Bioactive coating provides antimicrobial protection through immunomodulation and phage therapeutics
- PMID: 38525309
- PMCID: PMC10959705
- DOI: 10.1016/j.mtbio.2024.101022
Bioactive coating provides antimicrobial protection through immunomodulation and phage therapeutics
Abstract
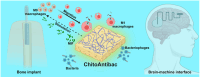
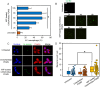
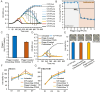
Medical implant-associated infections (IAI) is a growing threat to patients undergoing implantation surgery. IAI prevention typically relies on medical implants endowed with bactericidal properties achieved through surface modifications with antibiotics. However, the clinical efficacy of this traditional paradigm remains suboptimal, often necessitating revision surgery and posing potentially lethal consequences for patients. To bolster the existing anti-IAI arsenal, we propose herein a chitosan-based bioactive coating, i.e., ChitoAntibac, which exerts bacteria-inhibitory effects either through immune modulation or phage-directed microbial clearance, without relying on conventional antibiotics. The immuno-stimulating effects and phage-induced bactericidal properties can be tailored by engineering the loading dynamic of macrophage migration inhibitory factor (MIF), which polarizes macrophages towards the proinflammatory subtype (M1) with enhanced bacterial phagocytosis, and Staphylococcal Phage K, resulting in rapid and targeted pathogenic clearance (>99.99%) in less than 8 h. Our innovative antibacterial coating opens a new avenue in the pursuit of effective IAI prevention through immuno-stimulation and phage therapeutics.
Keywords: Bacteriophage; Bioactive coating; Immune modulation; Implant-associated infections; Medical implants.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

