Stepwise degradable PGA-SF core-shell electrospinning scaffold with superior tenacity in wetting regime for promoting bone regeneration
- PMID: 38525312
- PMCID: PMC10959703
- DOI: 10.1016/j.mtbio.2024.101023
Stepwise degradable PGA-SF core-shell electrospinning scaffold with superior tenacity in wetting regime for promoting bone regeneration
Abstract
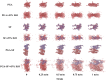
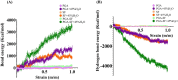
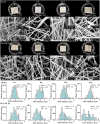
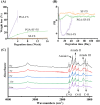
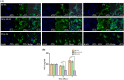
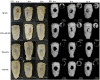
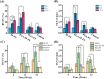
Regenerating bone in the oral and maxillofacial region is clinically challenging due to the complicated osteogenic environment and the limitation of existing bone graft materials. Constructing bone graft materials with controlled degradation and stable mechanical properties in a physiological environment is of utmost importance. In this study, we used silk fibroin (SF) and polyglycolic acid (PGA) to fabricate a coaxial PGA-SF fibrous scaffold (PGA-SF-FS) to meet demands for bone grafts. The SF shell exerted excellent osteogenic activity while protecting PGA from rapid degradation and the PGA core equipped scaffold with excellent tenacity. The experiments related to biocompatibility and osteogenesis (e.g., cell attachment, proliferation, differentiation, and mineralization) demonstrated the superior ability of PGA-SF-FS to improve cell growth and osteogenic differentiation. Furthermore, in vivo testing using Sprague-Dawley rat cranial defect model showed that PGA-SF-FS accelerates bone regeneration as the implantation time increases, and its stepwise degradation helps to match the remodeling kinetics of the host bone tissue. Besides, immunohistochemical staining of CD31 and Col-1 confirmed the ability of PGA-SF-FS to enhance revascularization and osteogenesis response. Our results suggest that PGA-SF-FS fully utilizing the advantages of both components, exhibites stepwise degradation and superior tenacity in wetting regime, making it a promising candidate in the treatment of bone defects.
Keywords: Bone regeneration; Core-shell; Electrospinning; Stepwise degradation; Tenacity.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
LinkOut - more resources
Full Text Sources

