RET Fusion Testing in Patients With NSCLC: The RETING Study
- PMID: 38525319
- PMCID: PMC10957499
- DOI: 10.1016/j.jtocrr.2024.100653
RET Fusion Testing in Patients With NSCLC: The RETING Study
Abstract
Introduction: RET inhibitors with impressive overall response rates are now available for patients with NSCLC, yet the identification of RET fusions remains a difficult challenge. Most guidelines encourage the upfront use of next-generation sequencing (NGS), or alternatively, fluorescence in situ hybridization (FISH) or reverse transcriptase-polymerase chain reaction (RT-PCR) when NGS is not possible or available. Taken together, the suboptimal performance of single-analyte assays to detect RET fusions, although consistent with the notion of encouraging universal NGS, is currently widening some of the clinical practice gaps in the implementation of predictive biomarkers in patients with advanced NSCLC.
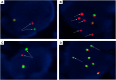
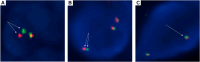
Methods: This situation prompted us to evaluate several RET assays in a large multicenter cohort of RET fusion-positive NSCLC (n = 38) to obtain real-world data. In addition to RNA-based NGS (the criterion standard method), all positive specimens underwent break-apart RET FISH with two different assays and were also tested by an RT-PCR assay.
Results: The most common RET partners were KIF5B (78.9%), followed by CCDC6 (15.8%). The two RET NGS-positive but FISH-negative samples contained a KIF5B(15)-RET(12) fusion. The three RET fusions not identified with RT-PCR were AKAP13(35)-RET(12), KIF5B(24)-RET(9) and KIF5B(24)-RET(11). All three false-negative RT-PCR cases were FISH-positive, exhibited a typical break-apart pattern, and contained a very high number of positive tumor cells with both FISH assays. Signet ring cells, psammoma bodies, and pleomorphic features were frequently observed (in 34.2%, 39.5%, and 39.5% of tumors, respectively).
Conclusions: In-depth knowledge of the advantages and disadvantages of the different RET testing methodologies could help clinical and molecular tumor boards implement and maintain sensible algorithms for the rapid and effective detection of RET fusions in patients with NSCLC. The likelihood of RET false-negative results with both FISH and RT-PCR reinforces the need for upfront NGS in patients with NSCLC.
Keywords: FISH; Lung carcinoma; Next-generation sequencing; RET fusions; RT-PCR.
© 2024 The Authors.
Conflict of interest statement
Dr. Conde has received research funding from Eli Lilly, 10.13039/100004325AstraZeneca, and 10.13039/100011033ThermoFisher Scientific; and honoraria from Pfizer, Roche, AstraZeneca, Janssen, and Eli Lilly. Dr. Hernandez has received research funding from Eli Lilly, AstraZeneca, and ThermoFisher Scientific, and honoraria from Pfizer, Roche, AstraZeneca, ThermoFisher Scientific, and Eli Lilly. Mr. Alonso has received research funding from AstraZeneca, and honoraria from Pfizer, Roche, and AstraZeneca. Dr. Jimenez has received honoraria from Roche. Dr. Garrido has received research grants from 10.13039/100002429Amgen, AstraZeneca, Blueprint, 10.13039/100002491Bristol-Myers Squibb, Boehringer Ingelheim, 10.13039/501100002973Daiichi-Sankyo, 10.13039/100004330GlaxoSmithKline, 10.13039/100005565Janssen, IO Biotech, Eli Lilly, 10.13039/100009947Merck Sharp & Dohme, 10.13039/100004337Roche, Takeda; and honoraria from AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, Takeda, Medscape, and Touch Medical. Dr. Clave has received honoraria from AstraZeneca, Pfizer, Roche, Eli Lilly, and Takeda. Dr. Arriola has received honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche/Genentech, Eli Lilly and Company, Novartis, Takeda, Merck Sharp & Dohme, Bayer, and Bristol Myers Squibb. Dr. Esteban-Rodriguez has received honoraria from AstraZeneca, Pfizer, and Merck Sharp & Dohme. Dr. De Castro has received honoraria from AstraZeneca, Bristol Myers Squibb, Hoffmann- La Roche, Merck Sharp and Dohme, Boehringer-Ingelheim, Janssen, Eli Lilly, Sanofi, Takeda, Pfizer, Glaxo, and Gilead. Dr. Sansano has received honoraria from F. Hoffmann La Roche AG, Merck Sharp & Dohme, Pfizer, Takeda, AstraZeneca, and Boehringer Ingelheim. Dr. Felip has received honoraria from AbbVie, Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eli Lilly, F. Hoffmann – La Roche, Gilead, Glaxo Smith Kline, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Peervoice, Pfizer, Regeneron, Sanofi, Takeda, Turning Point, and Touch Oncology. Dr. Rojo has received research funding from 10.13039/100004337Roche, AstraZeneca, Menarini, 10.13039/100004336Novartis, 10.13039/100004334Merck, Merck Sharp & Dohme, Bristol-Myers Squibb, 10.13039/100004319Pfizer, GlaxoSmithKline, Palex, Amgen, 10.13039/100004322Agilent, and Janssen, and honoraria from Roche, AstraZeneca, Menarini, Novartis, Merck, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Palex, Amgen, Agilent, Janssen. Dr. Dómine has received honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche/Genentech, Takeda, Merck Sharp & Dohme, and Bristol Myers Squibb. Dr. Abdulkader has received honoraria from AstraZeneca, Eli Lilly, Pfizer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Takeda, and Agilent Technologies S.A. Dr. Garcia-Gonzalez has received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Sanofi, Pierre Fabre, Eli Lilly, Pfizer, and Takeda. Dr. Teixido has received honoraria from Novartis, AstraZeneca, Roche, Merck Sharp Dohme, Pfizer, Janssen, Eli Lilly, and, Bristol Myers Squibb. Dr. Reguart has received honoraria from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer, Guardant, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Takeda. Dr. Insa has received honoraria from Roche, Bristol Myers Squibb, Sanofi, Pfizer, Boehringer Ingelheim, AstraZeneca, Takeda, Bayer, Merck Sharp & Dohme, and Eli Lilly. Dr. Mancheño has received honoraria from Roche, AstraZeneca, and Pfizer. Dr. Palanca has received honoraria from Roche Pharma, Pfizer, Amgen, AstraZeneca, Takeda, Eli Lilly, and Janssen. Dr. Juan-Vidal has received honoraria from Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genetech, AstraZeneca, Pfizer, Eli Lilly, and Takeda. Dr. Baixeras has received honoraria from AstraZeneca and Eli Lilly. Dr. Nadal has received research funding from Roche, Pfizer, Bristol-Myers Squibb and Merck Serono, and honoraria from Roche, Bristol Myers Squibb, Merck Sharp Dohme, Merck Serono, Sanofi, Pfizer, Eli Lilly, Janssen, Amgen, Daiichi-Sankyo, Boehringer Ingelheim, AstraZeneca, Takeda, Sanofi, Pierre Fabre, Qiagen, Janssen, and Bayer. Dr. Calles has received research funding from Merck Sharp & Dome, and honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche/Genentech, Eli Lilly and Company, Novartis, Takeda, Merck Sharp & Dohme, and Bristol Myers Squibb. Dr. Martin has received honoraria from Daiichi-Sankyo and Pfizer. Dr. Salas has received honoraria from Boehringer Ingelheim, Pfizer, and Merck Sharp & Dohme. Dr. Provencio has received honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche/Genentech, Takeda, Merck Sharp & Dohme, and Bristol Myers Squibb. Dr. Massuti has received research funding from Bristol Myers Squibb, and honoraria from Bristol Myers Squibb, Roche, Janssen, Merck Sharp & Dohme, and AstraZeneca. Dr. Majem has received research funding from 10.13039/100002429Amgen Inc., AstraZeneca, Bristol Myers Squibb, and Roche, and honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Kyowa Kyrin, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, Sanofi, and Takeda. Dr. Paz-Ares has received research funding from Merck Sharp & Dohme, AstraZeneca, Pfizer, and Bristol-Myers Squibb, and honoraria from Eli Lilly, Merck Sharp & Dohme, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, Bayer, Bristol-Myers Squibb, Mirati, GlaxoSmithKline, Janssen, Takeda, and Mirati. Dr. Lopez-Rios has received research funding from Eli Lilly, AstraZeneca, Roche, Pfizer, and ThermoFisher Scientific, and honoraria from Abbvie, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, Eli Lilly, Merck Sharp & Dohme, Merck, Pfizer, Roche, Sanofi, Takeda, and Thermo Fisher. The remaining authors declare no conflict of interest.
Figures


Similar articles
-
A Performance Comparison of Commonly Used Assays to Detect RET Fusions.Clin Cancer Res. 2021 Mar 1;27(5):1316-1328. doi: 10.1158/1078-0432.CCR-20-3208. Epub 2020 Dec 3. Clin Cancer Res. 2021. PMID: 33272981 Free PMC article.
-
Pitfalls in RET Fusion Detection Using Break-Apart FISH Probes in Papillary Thyroid Carcinoma.J Clin Endocrinol Metab. 2021 Mar 25;106(4):1129-1138. doi: 10.1210/clinem/dgaa913. J Clin Endocrinol Metab. 2021. PMID: 33382428
-
Detection of clinically actionable gene fusions by next-generation sequencing-based RNA sequencing of non-small cell lung cancer cytology specimens: A single-center experience with comparison to fluorescence in situ hybridization.Cancer Cytopathol. 2024 Jan;132(1):41-49. doi: 10.1002/cncy.22766. Epub 2023 Sep 25. Cancer Cytopathol. 2024. PMID: 37747438
-
A narrative review of methods for the identification of ALK fusions in patients with non-small cell lung carcinoma.Transl Lung Cancer Res. 2023 Jul 31;12(7):1549-1562. doi: 10.21037/tlcr-22-855. Epub 2023 Jul 11. Transl Lung Cancer Res. 2023. PMID: 37577307 Free PMC article. Review.
-
Will the Requirement by the US FDA to Simultaneously Co-Develop Companion Diagnostics (CDx) Delay the Approval of Receptor Tyrosine Kinase Inhibitors for RTK-Rearranged (ROS1-, RET-, AXL-, PDGFR-α-, NTRK1-) Non-Small Cell Lung Cancer Globally?Front Oncol. 2014 Apr 1;4:58. doi: 10.3389/fonc.2014.00058. eCollection 2014. Front Oncol. 2014. PMID: 24744988 Free PMC article. Review.
Cited by
-
Clinical utility of circulating tumor DNA profiling in detecting targetable fusions in non-small cell lung cancer.Front Oncol. 2024 Oct 24;14:1463341. doi: 10.3389/fonc.2024.1463341. eCollection 2024. Front Oncol. 2024. PMID: 39507756 Free PMC article.
References
-
- Lin J.J., Gainor J.F. Selective targeting of RET fusions in lung cancer. J Clin Oncol. 2023;41:410–412. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

