A simple solid media assay for detection of synergy between bacteriophages and antibiotics
- PMID: 38526142
- PMCID: PMC11064537
- DOI: 10.1128/spectrum.03221-23
A simple solid media assay for detection of synergy between bacteriophages and antibiotics
Abstract
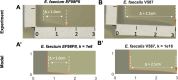
The emergence of antibiotic-resistant bacteria (ARB) has necessitated the development of alternative therapies to deal with this global threat. Bacteriophages (viruses that target bacteria) that kill ARB are one such alternative. Although phages have been used clinically for decades with inconsistent results, a number of recent advances in phage selection, propagation, and purification have enabled a reevaluation of their utility in contemporary clinical medicine. In most phage therapy cases, phages are administered in combination with antibiotics to ensure that patients receive the standard-of-care treatment. Some phages may work cooperatively with antibiotics to eradicate ARB, as often determined using non-standardized broth assays. We sought to develop a solid media-based assay to assess cooperativity between antibiotics and phages to offer a standardized platform for such testing. We modeled the interactions that occur between antibiotics and phages on solid medium to measure additive, antagonistic, and synergistic interactions. We then tested the method using different bacterial isolates and identified a number of isolates where synergistic interactions were identified. These interactions were not dependent on the specific organism, phage family, or antibiotic used. A priori susceptibility to the antibiotic or the specific phage were not requirements to observe synergistic interactions. Our data also confirm the potential for the restoration of vancomycin to treat vancomycin-resistant Enterococcus (VRE) when used in combination with phages. Solid media assays for the detection of cooperative interactions between antibiotics and phages can be an accessible technique adopted by clinical laboratories to evaluate antibiotic and phage choices in phage therapy.IMPORTANCEBacteriophages have become an important alternative treatment for individuals with life-threatening antibiotic-resistant bacteria (ARB) infections. Because antibiotics represent the standard-of-care for treatment of ARB, antibiotics and phages often are delivered together without evidence that they work cooperatively. Testing for cooperativity can be difficult due to the equipment necessary and a lack of standardized means for performing the testing in liquid medium. We developed an assay using solid medium to identify interactions between antibiotics and phages for gram-positive and gram-negative bacteria. We modeled the interactions between antibiotics and phages on solid medium, and then tested multiple replicates of vancomycin-resistant Enterococcus (VRE) and Stenotrophomonas in the assay. For each organism, we identified synergy between different phage and antibiotic combinations. The development of this solid media assay for assessing synergy between phages and antibiotics will better inform the use of these combinations in the treatment of ARB infections.
Keywords: antibiotics; bacteriophages; cooperativity; solid media; synergy.
Conflict of interest statement
S.I.F. is a scientific cofounder, director, and advisor of MelioLabs, Inc., and has an equity interest in the company. NIAID award number R01AI134982 has been identified for conflict-of-interest management based on the overall scope of the project and its potential benefit to MelioLabs, Inc.; however, the research findings included in this particular publication may not necessarily relate to the interests of MelioLabs, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict-of-interest policies.
Figures







Update of
-
A simple solid media assay for detection of synergy between bacteriophages and antibiotics.bioRxiv [Preprint]. 2023 Aug 24:2023.08.23.554535. doi: 10.1101/2023.08.23.554535. bioRxiv. 2023. Update in: Microbiol Spectr. 2024 May 2;12(5):e0322123. doi: 10.1128/spectrum.03221-23. PMID: 37662290 Free PMC article. Updated. Preprint.
References
-
- Aslam S, Lampley E, Wooten D, Karris M, Benson C, Strathdee S, Schooley RT. 2020. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 7:ofaa389. doi: 10.1093/ofid/ofaa389 - DOI - PMC - PubMed
-
- Morrisette T, Lev KL, Kebriaei R, Abdul-Mutakabbir JC, Stamper KC, Morales S, Lehman SM, Canfield GS, Duerkop BA, Arias CA, Rybak MJ. 2020. Bacteriophage-antibiotic combinations for Enterococcus faecium with varying bacteriophage and daptomycin susceptibilities. Antimicrob Agents Chemother 64:e00993-20. doi: 10.1128/AAC.00993-20 - DOI - PMC - PubMed
-
- Łusiak-Szelachowska M, Międzybrodzki R, Drulis-Kawa Z, Cater K, Knežević P, Winogradow C, Amaro K, Jończyk-Matysiak E, Weber-Dąbrowska B, Rękas J, Górski A. 2022. Bacteriophages and antibiotic interactions in clinical practice: what we have learned so far. J Biomed Sci 29:23. doi: 10.1186/s12929-022-00806-1 - DOI - PMC - PubMed

