Distinct features of the regenerating heart uncovered through comparative single-cell profiling
- PMID: 38526188
- PMCID: PMC11007736
- DOI: 10.1242/bio.060156
Distinct features of the regenerating heart uncovered through comparative single-cell profiling
Abstract
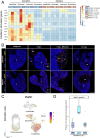
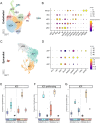
Adult humans respond to heart injury by forming a permanent scar, yet other vertebrates are capable of robust and complete cardiac regeneration. Despite progress towards characterizing the mechanisms of cardiac regeneration in fish and amphibians, the large evolutionary gulf between mammals and regenerating vertebrates complicates deciphering which cellular and molecular features truly enable regeneration. To better define these features, we compared cardiac injury responses in zebrafish and medaka, two fish species that share similar heart anatomy and common teleost ancestry but differ in regenerative capability. We used single-cell transcriptional profiling to create a time-resolved comparative cell atlas of injury responses in all major cardiac cell types across both species. With this approach, we identified several key features that distinguish cardiac injury response in the non-regenerating medaka heart. By comparing immune responses to injury, we found altered cell recruitment and a distinct pro-inflammatory gene program in medaka leukocytes, and an absence of the injury-induced interferon response seen in zebrafish. In addition, we found a lack of pro-regenerative signals, including nrg1 and retinoic acid, from medaka endothelial and epicardial cells. Finally, we identified alterations in the myocardial structure in medaka, where they lack primordial layer cardiomyocytes and fail to employ a cardioprotective gene program shared by regenerating vertebrates. Our findings reveal notable variation in injury response across nearly all major cardiac cell types in zebrafish and medaka, demonstrating how evolutionary divergence influences the hidden cellular features underpinning regenerative potential in these seemingly similar vertebrates.
Keywords: Evolution; Heart; Immunity; Interferon; Myocardium; Regeneration.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





Update of
-
Distinct features of the regenerating heart uncovered through comparative single-cell profiling.bioRxiv [Preprint]. 2023 Jul 12:2023.07.04.547574. doi: 10.1101/2023.07.04.547574. bioRxiv. 2023. Update in: Biol Open. 2024 Apr 15;13(4):bio060156. doi: 10.1242/bio.060156. PMID: 37461520 Free PMC article. Updated. Preprint.
Similar articles
-
Distinct features of the regenerating heart uncovered through comparative single-cell profiling.bioRxiv [Preprint]. 2023 Jul 12:2023.07.04.547574. doi: 10.1101/2023.07.04.547574. bioRxiv. 2023. Update in: Biol Open. 2024 Apr 15;13(4):bio060156. doi: 10.1242/bio.060156. PMID: 37461520 Free PMC article. Updated. Preprint.
-
Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration.Elife. 2017 Jun 20;6:e25605. doi: 10.7554/eLife.25605. Elife. 2017. PMID: 28632131 Free PMC article.
-
Myocardial NF-κB activation is essential for zebrafish heart regeneration.Proc Natl Acad Sci U S A. 2015 Oct 27;112(43):13255-60. doi: 10.1073/pnas.1511209112. Epub 2015 Oct 15. Proc Natl Acad Sci U S A. 2015. PMID: 26472034 Free PMC article.
-
Turning back the cardiac regenerative clock: lessons from the neonate.Trends Cardiovasc Med. 2012 Jul;22(5):128-33. doi: 10.1016/j.tcm.2012.07.008. Epub 2012 Aug 14. Trends Cardiovasc Med. 2012. PMID: 22902092 Review.
-
Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals.Biomolecules. 2020 Aug 19;10(9):1204. doi: 10.3390/biom10091204. Biomolecules. 2020. PMID: 32825069 Free PMC article. Review.
Cited by
-
Macrophages and cardiac lesion in zebrafish: what can single-cell RNA sequencing reveal?Front Cardiovasc Med. 2025 Apr 11;12:1570582. doi: 10.3389/fcvm.2025.1570582. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40290186 Free PMC article. Review.
-
Zebrafish as a Versatile Model for Cardiovascular Research: Peering into the Heart of the Matter.Cells. 2025 Apr 2;14(7):531. doi: 10.3390/cells14070531. Cells. 2025. PMID: 40214485 Free PMC article. Review.
-
Programmed cell revival from imminent cell death enhances tissue repair and regeneration.EMBO J. 2025 Aug 21. doi: 10.1038/s44318-025-00540-y. Online ahead of print. EMBO J. 2025. PMID: 40841709
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

