Novel Oral Poliovirus Vaccine 2 Safety Evaluation during Nationwide Supplemental Immunization Activity, Uganda, 2022
- PMID: 38526214
- PMCID: PMC10977820
- DOI: 10.3201/eid3004.231361
Novel Oral Poliovirus Vaccine 2 Safety Evaluation during Nationwide Supplemental Immunization Activity, Uganda, 2022
Abstract
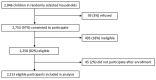
Given its enhanced genetic stability, novel oral poliovirus vaccine type 2 was deployed for type 2 poliovirus outbreak responses under World Health Organization Emergency Use Listing. We evaluated the safety profile of this vaccine. No safety signals were identified using a multipronged approach of passive and active surveillance.
Keywords: Uganda; poliovirus; vaccine safety; vaccines; viruses.
Figures
References
-
- Cooper LV, Bandyopadhyay AS, Gumede N, Mach O, Mkanda P, Ndoutabé M, et al. Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa. Lancet Infect Dis. 2022;22:284–94. 10.1016/S1473-3099(21)00453-9 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

