Ruxolitinib improves the inflammatory microenvironment, restores glutamate homeostasis, and promotes functional recovery after spinal cord injury
- PMID: 38526286
- PMCID: PMC11090442
- DOI: 10.4103/NRR.NRR-D-23-01863
Ruxolitinib improves the inflammatory microenvironment, restores glutamate homeostasis, and promotes functional recovery after spinal cord injury
Abstract
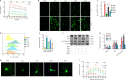
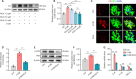
JOURNAL/nrgr/04.03/01300535-202419110-00030/figure1/v/2024-03-08T184507Z/r/image-tiff The inflammatory microenvironment and neurotoxicity can hinder neuronal regeneration and functional recovery after spinal cord injury. Ruxolitinib, a JAK-STAT inhibitor, exhibits effectiveness in autoimmune diseases, arthritis, and managing inflammatory cytokine storms. Although studies have shown the neuroprotective potential of ruxolitinib in neurological trauma, the exact mechanism by which it enhances functional recovery after spinal cord injury, particularly its effect on astrocytes, remains unclear. To address this gap, we established a mouse model of T10 spinal cord contusion and found that ruxolitinib effectively improved hindlimb motor function and reduced the area of spinal cord injury. Transcriptome sequencing analysis showed that ruxolitinib alleviated inflammation and immune response after spinal cord injury, restored EAAT2 expression, reduced glutamate levels, and alleviated excitatory toxicity. Furthermore, ruxolitinib inhibited the phosphorylation of JAK2 and STAT3 in the injured spinal cord and decreased the phosphorylation level of nuclear factor kappa-B and the expression of inflammatory factors interleukin-1β, interleukin-6, and tumor necrosis factor-α. Additionally, in glutamate-induced excitotoxicity astrocytes, ruxolitinib restored EAAT2 expression and increased glutamate uptake by inhibiting the activation of STAT3, thereby reducing glutamate-induced neurotoxicity, calcium influx, oxidative stress, and cell apoptosis, and increasing the complexity of dendritic branching. Collectively, these results indicate that ruxolitinib restores glutamate homeostasis by rescuing the expression of EAAT2 in astrocytes, reduces neurotoxicity, and effectively alleviates inflammatory and immune responses after spinal cord injury, thereby promoting functional recovery after spinal cord injury.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures






References
-
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. - PubMed
-
- Alijanpour S, Miryounesi M, Ghafouri-Fard S. The role of excitatory amino acid transporter 2 (EAAT2) in epilepsy and other neurological disorders. Metab Brain Dis. 2023;38:1–16. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Bretheau F, Castellanos-Molina A, Bélanger D, Kusik M, Mailhot B, Boisvert A, Vallières N, Lessard M, Gunzer M, Liu X, Boilard É, Quan N, Lacroix S. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat Commun. 2022;13:5786. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

