TMEM106B coding variant is protective and deletion detrimental in a mouse model of tauopathy
- PMID: 38526616
- PMCID: PMC12313335
- DOI: 10.1007/s00401-024-02701-5
TMEM106B coding variant is protective and deletion detrimental in a mouse model of tauopathy
Abstract
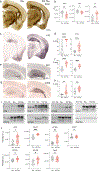
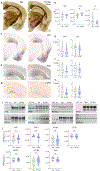
TMEM106B is a risk modifier of multiple neurological conditions, where a single coding variant and multiple non-coding SNPs influence the balance between susceptibility and resilience. Two key questions that emerge from past work are whether the lone T185S coding variant contributes to protection, and if the presence of TMEM106B is helpful or harmful in the context of disease. Here, we address both questions while expanding the scope of TMEM106B study from TDP-43 to models of tauopathy. We generated knockout mice with constitutive deletion of TMEM106B, alongside knock-in mice encoding the T186S knock-in mutation (equivalent to the human T185S variant), and crossed both with a P301S transgenic tau model to study how these manipulations impacted disease phenotypes. We found that TMEM106B deletion accelerated cognitive decline, hind limb paralysis, tau pathology, and neurodegeneration. TMEM106B deletion also increased transcriptional correlation with human AD and the functional pathways enriched in KO:tau mice aligned with those of AD. In contrast, the coding variant protected against tau-associated cognitive decline, synaptic impairment, neurodegeneration, and paralysis without affecting tau pathology. Our findings reveal that TMEM106B is a critical safeguard against tau aggregation, and that loss of this protein has a profound effect on sequelae of tauopathy. Our study further demonstrates that the coding variant is functionally relevant and contributes to neuroprotection downstream of tau pathology to preserve cognitive function.
Keywords: Alzheimer’s disease; Cognitive resilience; Frontotemporal dementia; Mouse model; Neurodegeneration; TMEM106B; Tau; Tauopathy.
© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Declarations
Figures





Update of
-
TMEM106B coding variant is protective and deletion detrimental in a mouse model of tauopathy.bioRxiv [Preprint]. 2023 Mar 25:2023.03.23.533978. doi: 10.1101/2023.03.23.533978. bioRxiv. 2023. Update in: Acta Neuropathol. 2024 Mar 25;147(1):61. doi: 10.1007/s00401-024-02701-5. PMID: 36993574 Free PMC article. Updated. Preprint.
References
-
- Briggs DI, Defensor E, Memar Ardestani P, Yi B, Halpain M, Seabrook G et al. (2017) Role of endoplasmic reticulum stress in learning and memory Impairment and Alzheimer’s disease-Like neuropathology in the PS19 and APP(Swe) mouse models of tauopathy and amyloidosis. ENeuro. 10.1523/ENEURO.0025-17.2017 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- F31 AG067676/AG/NIA NIH HHS/United States
- R01AG074009/NH/NIH HHS/United States
- S10 OD016167/OD/NIH HHS/United States
- P01 AG066606/AG/NIA NIH HHS/United States
- R01 AG074009/AG/NIA NIH HHS/United States
- R21AG056028/NH/NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- T32 NS043124/NS/NINDS NIH HHS/United States
- RF1 AG058188/AG/NIA NIH HHS/United States
- T32NS043124/NH/NIH HHS/United States
- P30 EY002520/EY/NEI NIH HHS/United States
- P01AG066606/NH/NIH HHS/United States
- P50 HD103555/HD/NICHD NIH HHS/United States
- RF1AG058188/NH/NIH HHS/United States
- R21 AG056028/AG/NIA NIH HHS/United States
- ZEN-19-591129/ALZ/Alzheimer's Association/United States
- WT_/Wellcome Trust/United Kingdom
- F31AG067676/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

