Secondary Sites of the C-type Lectin-Like Fold
- PMID: 38527187
- PMCID: PMC7618208
- DOI: 10.1002/chem.202400660
Secondary Sites of the C-type Lectin-Like Fold
Abstract
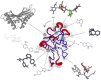
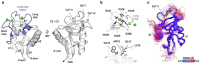
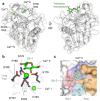
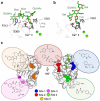
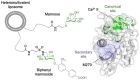
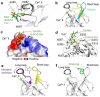
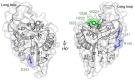
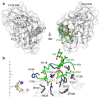
C-type lectins are a large superfamily of proteins involved in a multitude of biological processes. In particular, their involvement in immunity and homeostasis has rendered them attractive targets for diverse therapeutic interventions. They share a characteristic C-type lectin-like domain whose adaptability enables them to bind a broad spectrum of ligands beyond the originally defined canonical Ca2+-dependent carbohydrate binding. Together with variable domain architecture and high-level conformational plasticity, this enables C-type lectins to meet diverse functional demands. Secondary sites provide another layer of regulation and are often intricately linked to functional diversity. Located remote from the canonical primary binding site, secondary sites can accommodate ligands with other physicochemical properties and alter protein dynamics, thus enhancing selectivity and enabling fine-tuning of the biological response. In this review, we outline the structural determinants allowing C-type lectins to perform a large variety of tasks and to accommodate the ligands associated with it. Using the six well-characterized Ca2+-dependent and Ca2+-independent C-type lectin receptors DC-SIGN, langerin, MGL, dectin-1, CLEC-2 and NKG2D as examples, we focus on the characteristics of non-canonical interactions and secondary sites and their potential use in drug discovery endeavors.
© 2024 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of Glycobiology. 4th Cold Spring Harbor (NY); 2022. - PubMed
-
- Zelensky N, Gready JE. J Febs. 2005;272(24):6179–217. - PubMed
-
- McMahon SA, Miller JL, Lawton JA, Kerkow DE, Hodes A, Marti-Renom MA. Nat Struct Mol Biol. 2005;12(10):886–92. - PubMed
-
- Geijtenbeek TBH, Krooshoop DJEB, Bleijs DA, Vliet SJv, Grabovsky V, et al. Nat Immunol. 2000;1(4):353–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

