Type I Interferon, Induced by Adenovirus or Adenoviral Vector Infection, Regulates the Cytokine Response to Lipopolysaccharide in a Macrophage Type-Specific Manner
- PMID: 38527452
- PMCID: PMC11023693
- DOI: 10.1159/000538282
Type I Interferon, Induced by Adenovirus or Adenoviral Vector Infection, Regulates the Cytokine Response to Lipopolysaccharide in a Macrophage Type-Specific Manner
Abstract
Introduction: While TLR ligands derived from microbial flora and pathogens are important activators of the innate immune system, a variety of factors such as intracellular bacteria, viruses, and parasites can induce a state of hyperreactivity, causing a dysregulated and potentially life-threatening cytokine over-response upon TLR ligand exposure. Type I interferon (IFN-αβ) is a central mediator in the induction of hypersensitivity and is strongly expressed in splenic conventional dendritic cells (cDC) and marginal zone macrophages (MZM) when mice are infected with adenovirus. This study investigates the ability of adenoviral infection to influence the activation state of the immune system and underlines the importance of considering this state when planning the treatment of patients.
Methods: Infection with adenovirus-based vectors (Ad) or pretreatment with recombinant IFN-β was used as a model to study hypersensitivity to lipopolysaccharide (LPS) in mice, murine macrophages, and human blood samples. The TNF-α, IL-6, IFN-αβ, and IL-10 responses induced by LPS after pretreatment were measured. Mouse knockout models for MARCO, IFN-αβR, CD14, IRF3, and IRF7 were used to probe the mechanisms of the hypersensitive reaction.
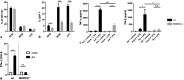
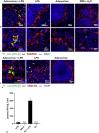
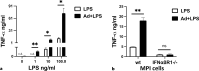
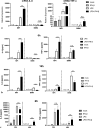
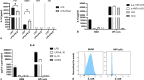
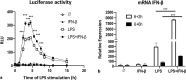
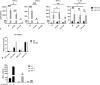
Results: We show that, similar to TNF-α and IL-6 but not IL-10, the induction of IFN-αβ by LPS increases strongly after Ad infection. This is true both in mice and in human blood samples ex vivo, suggesting that the regulatory mechanisms seen in the mouse are also present in humans. In mice, the scavenger receptor MARCO on IFN-αβ-producing cDC and splenic marginal zone macrophages is important for Ad uptake and subsequent cytokine overproduction by LPS. Interestingly, not all IFN-αβ-pretreated macrophage types exposed to LPS exhibit an enhanced TNF-α and IL-6 response. Pretreated alveolar macrophages and alveolar macrophage-like murine cell lines (MPI cells) show enhanced responses, while bone marrow-derived and peritoneal macrophages show a weaker response. This correlates with the respective absence or presence of the anti-inflammatory IL-10 response in these different macrophage types. In contrast, Ad or IFN-β pretreatment enhances the subsequent induction of IFN-αβ in all macrophage types. IRF3 is dispensable for the LPS-induced IFN-αβ overproduction in infected MPI cells and partly dispensable in infected mice, while IRF7 is required. The expression of the LPS co-receptor CD14 is important but not absolutely required for the elicitation of a TNF-α over-response to LPS in Ad-infected mice.
Conclusion: Viral infections or application of virus-based vaccines induces type I interferon and can tip the balance of the innate immune system in the direction of hyperreactivity to a subsequent exposure to TLR ligands. The adenoviral model presented here is one example of how multiple factors, both environmental and genetic, affect the physiological responses to pathogens. Being able to measure the current reactivity state of the immune system would have important benefits for infection-specific therapies and for the prevention of vaccination-elicited adverse effects.
Keywords: Adenoviral vector; Cytokines; IFN-αβ; Lipopolysaccharide; Macrophages.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
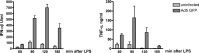


Similar articles
-
Absence of c-Maf and IL-10 enables type I IFN enhancement of innate responses to LPS in alveolar macrophages.J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. J Immunol. 2025. PMID: 40073087
-
Reconstitution of interferon regulatory factor 7 expression restores interferon beta induction in Huh7 cells.J Virol. 2025 Jun 17;99(6):e0070325. doi: 10.1128/jvi.00703-25. Epub 2025 May 23. J Virol. 2025. PMID: 40407345 Free PMC article.
-
Dual nature of type I interferon responses and feedback regulations by SOCS1 dictate malaria mortality.J Adv Res. 2025 Jul;73:295-310. doi: 10.1016/j.jare.2024.08.027. Epub 2024 Aug 22. J Adv Res. 2025. PMID: 39181199 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
Cited by
-
Absence of c-Maf and IL-10 enables type I IFN enhancement of innate responses to LPS in alveolar macrophages.J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. J Immunol. 2025. PMID: 40073087
-
Absence of c-Maf and IL-10 enables Type I IFN enhancement of innate responses to low-dose LPS in alveolar macrophages.bioRxiv [Preprint]. 2024 May 26:2024.05.22.594428. doi: 10.1101/2024.05.22.594428. bioRxiv. 2024. Update in: J Immunol. 2025 Mar 1;214(3):551-564. doi: 10.1093/jimmun/vkae029. PMID: 38826239 Free PMC article. Updated. Preprint.
References
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. - PubMed
-
- Galanos C, Freudenberg MA, Matsuura M, Coumbos A. Hypersensitivity to endotoxin and mechanisms of host-response. Prog Clin Biol Res. 1988;272:295–308. - PubMed
-
- Freudenberg MA, Kalis C, Chvatchko Y, Merlin T, Gumenscheimer M, Galanos C. Role of interferons in LPS hypersensitivity. J Endotoxin Res. 2003;9(5):308–12. - PubMed
-
- Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schütze N, et al. . Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213(3–4):193–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

