Inhibition of JAK-STAT pathway corrects salivary gland inflammation and interferon driven immune activation in Sjögren's disease
- PMID: 38527764
- PMCID: PMC11250564
- DOI: 10.1136/ard-2023-224842
Inhibition of JAK-STAT pathway corrects salivary gland inflammation and interferon driven immune activation in Sjögren's disease
Abstract
Objectives: Inflammatory cytokines that signal through the Janus kinases-signal transducer and activator of transcription (JAK-STAT) pathway, especially interferons (IFNs), are implicated in Sjögren's disease (SjD). Although inhibition of JAKs is effective in other autoimmune diseases, a systematic investigation of IFN-JAK-STAT signalling and the effect of JAK inhibitor (JAKi) therapy in SjD-affected human tissues has not been fully investigated.
Methods: Human minor salivary glands (MSGs) and peripheral blood mononuclear cells (PBMCs) were investigated using bulk or single-cell (sc) RNA sequencing (RNAseq), immunofluorescence (IF) microscopy and flow cytometry. Ex vivo culture assays on PBMCs and primary salivary gland epithelial cell (pSGEC) lines were performed to model changes in target tissues before and after JAKi.
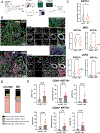
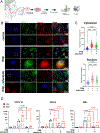
Results: RNAseq and IF showed activated JAK-STAT pathway in SjD MSGs. Elevated IFN-stimulated gene (ISGs) expression associated with clinical variables (eg, focus scores, anti-SSA positivity). scRNAseq of MSGs exhibited cell type-specific upregulation of JAK-STAT and ISGs; PBMCs showed similar trends, including markedly upregulated ISGs in monocytes. Ex vivo studies showed elevated basal pSTAT levels in SjD MSGs and PBMCs that were corrected with JAKi. SjD-derived pSGECs exhibited higher basal ISG expressions and exaggerated responses to IFN-β, which were normalised by JAKi without cytotoxicity.
Conclusions: SjD patients' tissues exhibit increased expression of ISGs and activation of the JAK-STAT pathway in a cell type-dependent manner. JAKi normalises this aberrant signalling at the tissue level and in PBMCs, suggesting a putative viable therapy for SjD, targeting both glandular and extraglandular symptoms. Predicated on these data, a phase Ib/IIa randomised controlled trial to treat SjD with tofacitinib was initiated.
Keywords: Antirheumatic Agents; Autoimmune Diseases; Cytokines; Sjogren's Syndrome.
© Author(s) (or their employer(s)) 2024. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BMW has Cooperative Research Award and Development Agreements (CRADA) from Pfizer and Mitobridge (a subsidiary of Astellas Pharma). NIAMS has CRADAs with AstraZeneca and Bristol Myers Squibb. These CRADA did not financially support the experimental results presented herein.
Figures





Update of
-
Inhibition of JAK-STAT pathway corrects salivary gland inflammation and interferon driven immune activation in Sjögren's Disease.medRxiv [Preprint]. 2023 Aug 21:2023.08.16.23294130. doi: 10.1101/2023.08.16.23294130. medRxiv. 2023. Update in: Ann Rheum Dis. 2024 Jul 15;83(8):1034-1047. doi: 10.1136/ard-2023-224842. PMID: 37662351 Free PMC article. Updated. Preprint.
References
-
- van Nimwegen JF, Mossel E, van Zuiden GS, et al. Abatacept treatment for patients with early active primary Sjögren’s syndrome: a single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study). The Lancet Rheumatology 2020;2(3):e153–e63. doi: 10.1016/S2665-9913(19)30160-2 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

