SKA2 regulated hyperactive secretory autophagy drives neuroinflammation-induced neurodegeneration
- PMID: 38528004
- PMCID: PMC10963788
- DOI: 10.1038/s41467-024-46953-x
SKA2 regulated hyperactive secretory autophagy drives neuroinflammation-induced neurodegeneration
Abstract
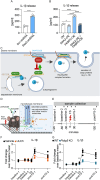
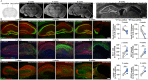
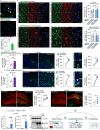
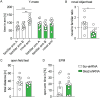
High levels of proinflammatory cytokines induce neurotoxicity and catalyze inflammation-driven neurodegeneration, but the specific release mechanisms from microglia remain elusive. Here we show that secretory autophagy (SA), a non-lytic modality of autophagy for secretion of vesicular cargo, regulates neuroinflammation-mediated neurodegeneration via SKA2 and FKBP5 signaling. SKA2 inhibits SA-dependent IL-1β release by counteracting FKBP5 function. Hippocampal Ska2 knockdown in male mice hyperactivates SA resulting in neuroinflammation, subsequent neurodegeneration and complete hippocampal atrophy within six weeks. The hyperactivation of SA increases IL-1β release, contributing to an inflammatory feed-forward vicious cycle including NLRP3-inflammasome activation and Gasdermin D-mediated neurotoxicity, which ultimately drives neurodegeneration. Results from protein expression and co-immunoprecipitation analyses of male and female postmortem human brains demonstrate that SA is hyperactivated in Alzheimer's disease. Overall, our findings suggest that SKA2-regulated, hyperactive SA facilitates neuroinflammation and is linked to Alzheimer's disease, providing mechanistic insight into the biology of neuroinflammation.
© 2024. The Author(s).
Conflict of interest statement
N.D. is currently an employee of Sumitomo Pharma America. K.M.M. is currently an employee of Jazz Pharmaceuticals. S.M. is currently an employee of Roche Diagnostics. Their contributions pre-date that employment and their co-authorship do not reflect collaboration on this project with these organizations. K.J.R. has received consulting income from Alkermes, Bionomics, and BioXcel and is on scientific advisory boards for Janssen and Verily for unrelated work. He has also received a sponsored research grant support from Takeda, Alto Neuroscience, and Brainsway for unrelated work. T.K. has received consulting income from Alkermes for unrelated work. Within the last 2 years, W.A.C. has served as a consultant for Psy Therapeutics and has had sponsored research agreements with Cerevel Therapeutics and Delix Therapeutics. The remaining authors declare no competing interests.
Figures


Update of
-
SKA2 regulated hyperactive secretory autophagy drives neuroinflammation-induced neurodegeneration.bioRxiv [Preprint]. 2023 Apr 3:2023.04.03.534570. doi: 10.1101/2023.04.03.534570. bioRxiv. 2023. Update in: Nat Commun. 2024 Mar 25;15(1):2635. doi: 10.1038/s41467-024-46953-x. PMID: 37066393 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

