Comparative response to PDT with methyl-aminolevulinate and temoporfin in cutaneous and oral squamous cell carcinoma cells
- PMID: 38528037
- PMCID: PMC10963768
- DOI: 10.1038/s41598-024-57624-8
Comparative response to PDT with methyl-aminolevulinate and temoporfin in cutaneous and oral squamous cell carcinoma cells
Abstract
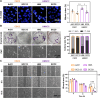
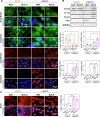
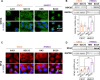
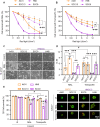
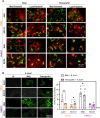
Cutaneous and Head and Neck squamous cell carcinoma (CSCC, HNSCC) are among the most prevalent cancers. Both types of cancer can be treated with photodynamic therapy (PDT) by using the photosensitizer Temoporfin in HNSCC and the prodrug methyl-aminolevulinate (MAL) in CSCC. However, PDT is not always effective. Therefore, it is mandatory to correctly approach the therapy according to the characteristics of the tumour cells. For this reason, we have used cell lines of CSCC (A431 and SCC13) and HNSCC (HN5 and SCC9). The results obtained indicated that the better response to MAL-PDT was related to its localization in the plasma membrane (A431 and HN5 cells). However, with Temoporfin all cell lines showed lysosome localization, even the most sensitive ones (HN5). The expression of mesenchymal markers and migratory capacity was greater in HNSCC lines compared to CSCC, but no correlation with PDT response was observed. The translocation to the nucleus of β-catenin and GSK3β and the activation of NF-κβ is related to the poor response to PDT in the HNSCC lines. Therefore, we propose that intracellular localization of GSK3β could be a good marker of response to PDT in HNSCC. Although the molecular mechanism of response to PDT needs further elucidation, this work shows that the most MAL-resistant line of CSCC is more sensitive to Temoporfin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Temoporfin-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: A multicenter study.Head Neck. 2010 Dec;32(12):1597-604. doi: 10.1002/hed.21368. Head Neck. 2010. PMID: 20848401 Clinical Trial.
-
Effects of photodynamic therapy using Red LED-light combined with hypocrellin B on apoptotic signaling in cutaneous squamous cell carcinoma A431 cells.Photodiagnosis Photodyn Ther. 2023 Sep;43:103683. doi: 10.1016/j.pdpdt.2023.103683. Epub 2023 Jun 28. Photodiagnosis Photodyn Ther. 2023. PMID: 37390854
-
Effect of Methyl Aminolevulinate Photodynamic Therapy With and Without Ablative Fractional Laser Treatment in Patients With Microinvasive Squamous Cell Carcinoma: A Randomized Clinical Trial.JAMA Dermatol. 2017 Mar 1;153(3):289-295. doi: 10.1001/jamadermatol.2016.4463. JAMA Dermatol. 2017. PMID: 28199463 Clinical Trial.
-
Photodynamic therapy for the treatment of microinvasive squamous cell carcinoma of the lower lip: a case report.G Ital Dermatol Venereol. 2015 Jun;150(3):331-5. Epub 2014 Jun 30. G Ital Dermatol Venereol. 2015. PMID: 24975947 Review.
-
Photodynamic therapy with methyl-5-aminolevulinate for basal cell carcinoma: A systematic review and meta-analysis.Photodiagnosis Photodyn Ther. 2020 Mar;29:101667. doi: 10.1016/j.pdpdt.2020.101667. Epub 2020 Jan 22. Photodiagnosis Photodyn Ther. 2020. PMID: 31978564
References
MeSH terms
Substances
Grants and funding
- PI18/00708/Instituto de Salud Carlos III MINECO and Feder Funds
- PI21/00953/Instituto de Salud Carlos III MINECO and Feder Funds
- PI21/00953/Instituto de Salud Carlos III MINECO and Feder Funds
- PI18/00858/Instituto de Salud Carlos III MINECO and Feder Funds
- PI18/00708/Instituto de Salud Carlos III MINECO and Feder Funds
LinkOut - more resources
Full Text Sources
Medical
Research Materials

