TOLLIP inhibits lipid accumulation and the integrated stress response in alveolar macrophages to control Mycobacterium tuberculosis infection
- PMID: 38528148
- PMCID: PMC11034867
- DOI: 10.1038/s41564-024-01641-w
TOLLIP inhibits lipid accumulation and the integrated stress response in alveolar macrophages to control Mycobacterium tuberculosis infection
Abstract
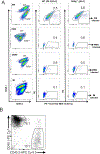
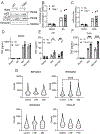
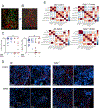
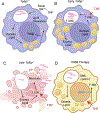
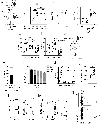
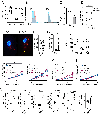
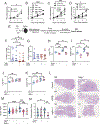
A polymorphism causing deficiencies in Toll-interacting protein (TOLLIP), an inhibitory adaptor protein affecting endosomal trafficking, is associated with increased tuberculosis (TB) risk. It is, however, unclear how TOLLIP affects TB pathogenesis. Here we show that TB severity is increased in Tollip-/- mice, characterized by macrophage- and T cell-driven inflammation, foam cell formation and lipid accumulation. Tollip-/- alveolar macrophages (AM) specifically accumulated lipid and underwent necrosis. Transcriptional and protein analyses of Mycobacterium tuberculosis (Mtb)-infected, Tollip-/- AM revealed increased EIF2 signalling and downstream upregulation of the integrated stress response (ISR). These phenotypes were linked, as incubation of the Mtb lipid mycolic acid with Mtb-infected Tollip-/- AM activated the ISR and increased Mtb replication. Correspondingly, the ISR inhibitor, ISRIB, reduced Mtb numbers in AM and improved Mtb control, overcoming the inflammatory phenotype. In conclusion, targeting the ISR offers a promising target for host-directed anti-TB therapy towards improved Mtb control and reduced immunopathology.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures


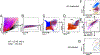




References
-
- Uhlén M et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- L30 AI091018/AI/NIAID NIH HHS/United States
- S10 OD024979/OD/NIH HHS/United States
- R03 HL155075/HL/NHLBI NIH HHS/United States
- R01 DK108921/DK/NIDDK NIH HHS/United States
- K08 AI166072/AI/NIAID NIH HHS/United States
- K08 AI102971/AI/NIAID NIH HHS/United States
- R01 DK135268/DK/NIDDK NIH HHS/United States
- U01 DK127747/DK/NIDDK NIH HHS/United States
- I01 BX004444/BX/BLRD VA/United States
- R01 AI136921/AI/NIAID NIH HHS/United States
- R01 DK136671/DK/NIDDK NIH HHS/United States
- R01 HL166273/HL/NHLBI NIH HHS/United States
- R01 CA136912/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

