Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation
- PMID: 38528202
- PMCID: PMC11088999
- DOI: 10.1038/s41593-024-01604-8
Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation
Abstract
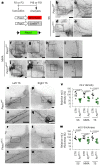
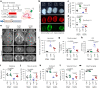
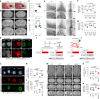
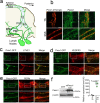
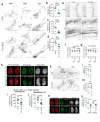
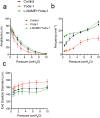
Piezo1 regulates multiple aspects of the vascular system by converting mechanical signals generated by fluid flow into biological processes. Here, we find that Piezo1 is necessary for the proper development and function of meningeal lymphatic vessels and that activating Piezo1 through transgenic overexpression or treatment with the chemical agonist Yoda1 is sufficient to increase cerebrospinal fluid (CSF) outflow by improving lymphatic absorption and transport. The abnormal accumulation of CSF, which often leads to hydrocephalus and ventriculomegaly, currently lacks effective treatments. We discovered that meningeal lymphatics in mouse models of Down syndrome were incompletely developed and abnormally formed. Selective overexpression of Piezo1 in lymphatics or systemic administration of Yoda1 in mice with hydrocephalus or Down syndrome resulted in a notable decrease in pathological CSF accumulation, ventricular enlargement and other associated disease symptoms. Together, our study highlights the importance of Piezo1-mediated lymphatic mechanotransduction in maintaining brain fluid drainage and identifies Piezo1 as a promising therapeutic target for treating excessive CSF accumulation and ventricular enlargement.
© 2024. The Author(s).
Conflict of interest statement
The authors disclose no conflict of interest with this study except that The University of Southern California has filed a patent application, entitled ‘Methods and compositions for fluid drainage by Piezo ion channel activation’ on behalf of Y.-K.H. and D.C.
Figures












References
-
- Ge J, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

