Early and Sustained Improvements in Symptoms and Quality of Life with Upadacitinib in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: 52-Week Results from Two Phase III Randomized Clinical Trials (Measure Up 1 and Measure Up 2)
- PMID: 38528257
- PMCID: PMC11070400
- DOI: 10.1007/s40257-024-00853-4
Early and Sustained Improvements in Symptoms and Quality of Life with Upadacitinib in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: 52-Week Results from Two Phase III Randomized Clinical Trials (Measure Up 1 and Measure Up 2)
Abstract
Background: Atopic dermatitis is a chronic inflammatory disease characterized by increased itch, skin pain, poor sleep quality, and other symptoms that negatively affect patient quality of life. Upadacitinib, an oral selective Janus kinase (JAK) inhibitor with greater inhibitory potency for JAK1 than JAK2, JAK3, or tyrosine kinase 2, is approved to treat moderate-to-severe atopic dermatitis.
Objective: We aimed to evaluate the effect of upadacitinib on patient-reported outcomes over 52 weeks in adults and adolescents with moderate-to-severe atopic dermatitis.
Methods: Data from two phase III monotherapy trials of upadacitinib (Measure Up 1, NCT03569293; Measure Up 2, NCT03607422) were integrated. Changes in pruritus, pain, other skin symptoms, sleep, quality of life, mental health, and patient impression were evaluated. Patient-reported outcome assessments included the Worst Pruritus Numerical Rating Scale, Patient-Oriented Eczema Measure, Dermatology Life Quality Index, Atopic Dermatitis Symptom Scale, Atopic Dermatitis Impact Scale, Hospital Anxiety and Depression Scale, SCORing Atopic Dermatitis index, Patient Global Impression of Severity, Patient Global Impression of Change, and Patient Global Impression of Treatment. Minimal clinically important differences, achievement of scores representing minimal disease burden, and the change from baseline were evaluated in patients who received upadacitinib through week 52 and in patients who received placebo through week 16.
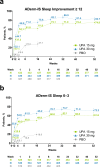
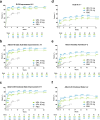
Results: This analysis included 1609 patients (upadacitinib 15 mg, N = 557; upadacitinib 30 mg, N = 567; placebo, N = 485). Baseline demographics and disease characteristics were generally similar across all arms. The proportion of patients treated with upadacitinib reporting improvements in itch increased rapidly by week 1, increased steadily through week 8, and was sustained through week 52. Patients receiving upadacitinib also experienced improvements in pain and other skin symptoms by week 1, which continued through week 16; improvements were maintained through week 52. Patient reports of improved sleep increased rapidly from baseline to week 1, increased steadily through week 32, and were sustained through week 52. Patients experienced quality-of-life improvements through week 8, which were maintained through week 52. By week 1, patients in both upadacitinib groups experienced rapid improvements in emotional state, and by week 12, patients also achieved meaningful improvements in anxiety and depression. Improvements in mental health continued steadily through week 32 and were maintained through week 52. Patients treated with upadacitinib 30 mg generally experienced improvements in patient-reported outcomes earlier than those treated with upadacitinib 15 mg. Through week 16, patients receiving upadacitinib experienced greater improvements versus those receiving placebo in all assessed patient-reported outcomes.
Conclusions: Adults and adolescents with moderate-to-severe atopic dermatitis treated with once-daily upadacitinib 15 or 30 mg experienced early improvements in itch, pain, other skin symptoms, sleep, quality of life, and mental health that were sustained through week 52.
Clinical trial registration: ClinicalTrials.gov identifiers NCT03569293 (13 August 2018) and NCT03607422 (27 July 2018).
Plain language summary
Atopic dermatitis, or eczema, is a condition that causes painful itchy dry skin, which is burdensome for patients and has a negative impact on quality of life. These symptoms frequently lead to disruption of daily activities such as school and work, decreased self-confidence, social isolation, anxiety, depression, and sleep disturbance. Symptoms of atopic dermatitis, such as itch and sleep disturbance, can only be assessed by patients. Therefore, it is important to consider patients’ perceptions of their symptoms and the related impact on their quality of life, especially when evaluating treatment benefits. Upadacitinib is an orally administered drug approved to treat moderate-to-severe atopic dermatitis. In two clinical trials (Measure Up 1 and Measure Up 2), we investigated how treatment with upadacitinib (15-mg or 30-mg dose) given once daily to adults and adolescents with moderate-to-severe atopic dermatitis would impact their symptoms and quality of life over a 1-year period. We measured changes over time in patients’ assessments of itch, pain, other skin-related symptoms, sleep, daily activities, emotional state, mental health, and overall quality of life. Patients treated with upadacitinib experienced improvements in symptoms of atopic dermatitis and quality of life within the first 1–2 weeks of treatment. These improvements continued to steadily increase in the following weeks and lasted through 1 year of treatment. In conclusion, once-daily treatment with upadacitinib 15 or 30 mg led to early and lasting improvements in the well-being of patients with atopic dermatitis.
© 2024. The Author(s).
Conflict of interest statement
Jonathan I. Silverberg receives consulting fees from AbbVie, Anacor Pharmaceuticals, GlaxoSmithKline, Eli Lilly, Regeneron Pharmaceuticals, Pfizer, Procter & Gamble, and MedImmune. He serves as an investigator in trials sponsored by Celgene, GlaxoSmithKline, Eli Lilly, Regeneron Pharmaceuticals, and Roche. Melinda J. Gooderham has been an investigator, speaker and/or advisor for AbbVie, Amgen, Akros, Arcutis, AnaptysBio, Apogee, Aristea, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Meiji, Merck, Moonlake, Nimbus, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Tarsus, Takeda, UCB, Union, and Ventyx. Amy S. Paller has been an investigator for AbbVie, Applied Pharma Research, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, Regeneron, and UCB; has been a consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Castle Creek, Eli Lilly, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi Genzyme, Seanergy, TWI Biotechnology, and UCB; and has served on the data safety monitoring board for AbbVie, Abeona, Catawba, Galderma, and InMed. Mette Deleuran receives consulting, lecturer, and/or investigator fees from AbbVie, Almirall, Arena Pharmaceuticals, ASLAN Pharmaceuticals, Eli Lilly, Incyte, Kymab, La Roche Posay, LEO Pharma, Numab, Pfizer, Pierre Fabre Laboratories, Regeneron Pharmaceuticals, and Sanofi. Christopher G. Bunick has served as an investigator for Almirall, Palvella, and Timber; a consultant for AbbVie, Almirall, Amgen, Apogee, Arcutis, Bristol Myers Squibb, Eli Lilly, LEO Pharma, Novan, Novartis, Ortho-Dermatologics, Pfizer, Sanofi-Regeneron, and UCB; and a speaker for and received honoraria from Allergan, Almirall, LEO Pharma, and UCB. Linda F. Stein Gold has served as an investigator/consultant or speaker for AbbVie, Arcutis, Dermavant, Incyte, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB. DirkJan Hijnen has served as an investigator, consultant, and/or speaker for AbbVie, Almirall, AstraZeneca, Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, and Sanofi. Brian M. Calimlim, Wan-Ju Lee, Henrique D. Teixeira, Xiaofei Hu, Shiyu Zhang, Yang Yang, Ayman Grada, and Andrew M. Platt are full-time employees of AbbVie, and may hold AbbVie stock or stock options. Diamant Thaçi is an advisor, speaker, or consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Galderma, Janssen, Kyowa Kirin, L'Oréal, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi/Genzyme, and UCB.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

