Hypersensitivity Reactions to Taxanes: A Multicenter Study for Outcomes and Safety of Rapid Drug Desensitization
- PMID: 38528382
- PMCID: PMC10973638
- DOI: 10.4168/aair.2024.16.2.142
Hypersensitivity Reactions to Taxanes: A Multicenter Study for Outcomes and Safety of Rapid Drug Desensitization
Abstract
Purpose: Taxanes can cause hypersensitivity reactions (HSRs), which pose a significant challenge in the treatment of malignancies. Patients who are eligible for rapid drug desensitization (RDD) can continue treatment; however, some patients experience breakthrough reactions (BTRs). Data about risk factors for BTRs during RDDs in patients with HSRs to taxanes are limited.
Methods: This was a multicenter, retrospective study of patients with immediate-HSRs to taxanes. Initial HSRs were classified as grade 1, 2, or 3 based on severity. Prick/intradermal skin tests were performed with implicated taxanes. A 12-step protocol was used during RDD.
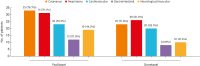
Results: The study comprised 75 patients (F/M: 63/12, mean age 49.92 ± 11.72 years, 43 HSRs to paclitaxel, 32 HSRs to docetaxel). The majority of reactions (86.7%) occurred during the first or second exposure. The prevalence of drug allergy history was higher in patients with paclitaxel HSR than in those with docetaxel HSR, although it was not statistically significant (23.3% vs. 6.3%). The initial HSRs were mostly grade 2 (n = 50, 66.7%) or grade 3 (n = 22, 29.3%). Skin tests with implicated taxanes were done on 48 patients, and the rate of positive response in patients with grade 1, 2, and 3 initial HSRs were 50%, 17.6%, and 16.7%, respectively. . A total of 255 RDDs were completely performed, although BTRs occurred in 27 (grade 1, 55.6%; grade 2, 40.7%; grade 3, 3.7%). There were no statistically significant correlations between the risk of BTR and age, drug cycle, gender, positivity of skin test or atopy. The step reduction was successfully done on 9 eligible patients with mild or moderate HSRs during the 12-step RDDs.
Conclusions: Our experience demonstrates a 100% success rate in completing the 255 RDDs for taxanes, affirming the safety and efficacy of the RDD within the study population.
Keywords: Desensitization; chemotherapy; docetaxel; drug hypersensitivity; neoplasms; paclitaxel; safety; taxoids.
Copyright © 2024 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures


References
-
- Mezzano V, Giavina-Bianchi P, Picard M, Caiado J, Castells M. Drug desensitization in the management of hypersensitivity reactions to monoclonal antibodies and chemotherapy. BioDrugs. 2014;28:133–144. - PubMed
-
- Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7:618–632. - PubMed
-
- Joerger M. Treatment regimens of classical and newer taxanes. Cancer Chemother Pharmacol. 2016;77:221–233. - PubMed
-
- Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA, Jr, Long AA. Management of hypersensitivity reactions to Carboplatin and Paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2:428–433. - PubMed
-
- Ribeiro-Vaz I, Marques J, Demoly P, Polónia J, Gomes ER. Drug-induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol. 2013;69:673–681. - PubMed

