Factors associated with vertical transmission of HIV in the Western Cape, South Africa: a retrospective cohort analysis
- PMID: 38528395
- PMCID: PMC10963590
- DOI: 10.1002/jia2.26235
Factors associated with vertical transmission of HIV in the Western Cape, South Africa: a retrospective cohort analysis
Abstract
Introduction: Monitoring mother-infant pairs with HIV exposure is needed to assess the effectiveness of vertical transmission (VT) prevention programmes and progress towards VT elimination.
Methods: We used routinely collected data on infants with HIV exposure, born May 2018-April 2021 in the Western Cape, South Africa, with follow-up through mid-2022. We assessed the proportion of infants diagnosed with HIV at birth (≤7 days), 10 weeks (>1 to 14 weeks) and >14 weeks as proxies for intrauterine, intrapartum/early breastfeeding and late breastfeeding transmission, respectively. We used mixed-effects Poisson regression to assess factors associated with VT in mothers known with HIV by delivery.
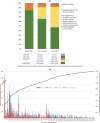
Results: We included 50,461 infants born to mothers known with HIV by delivery. HIV was diagnosed in 894 (1.8%) infants. Among mothers, 51% started antiretroviral treatment (ART) before and 27% during pregnancy; 17% restarted during pregnancy after ≥6 months interruption; and 6% had no recorded ART during pregnancy. Most pregnancy ART regimens included non-nucleoside reverse transcriptase inhibitors (83%). Of mothers with available results (90% with viral load [VL]; 70% with CD4), VL nearest delivery was <100 copies/ml in 78% and CD4 count ≥350 cells/μl in 62%. HIV-PCR results were available for 86%, 67% and 48% of eligible infants at birth, 10 weeks and >14 weeks. Among these infants, 0.9%, 0.4% and 1.5% were diagnosed positive at birth, 10 weeks and >14 weeks, respectively. Among infants diagnosed with HIV, 43%, 16% and 41% were diagnosed at these respective time periods. Among mothers with VL<100, 100-999, 1000-99,000 and ≥100,000 copies/ml nearest delivery, infant HIV diagnosis incidence was 0.4%, 2.3%, 6.6% and 18.4%, respectively. Increased VT was strongly associated with recent elevated maternal VL with a seven-fold increased rate with even modestly elevated VL (100-999 vs. <100 copies/ml). VT was also associated with unknown/low maternal CD4, maternal age <20 years, no antenatal ART, later maternal ART start/restart in pregnancy and ART gaps.
Conclusions: Despite high maternal ART coverage and routine postnatal prophylaxis, ongoing VT remains a concern. Timing of infant HIV diagnoses suggests intrapartum and/or breastfeeding transmission in nearly 60%. Interventions to ensure retention on ART and sustained maternal viral suppression are needed to reduce VT.
Keywords: HIV acquisitions; antiretroviral therapy; breastfeeding; infant; pregnancy; vertical transmission.
© 2024 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
KA, M‐AD and EK received funding from ViiV Healthcare for this project. The other authors have no competing interests to declare.
Figures
References
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed [Internet]. 2016. [cited 2022 Dec 11]. Available from: https://www.who.int/publications/i/item/9789241549684 - PubMed
-
- Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. - PubMed
-
- UNICEF . On the fast‐track to an AIDS‐free generation [Internet]. 2016. [cited 2022 Dec 11]. Available from: https://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en...
-
- World Health Organization . Global guidance on criteria and processes for validation: elimination of mother‐to‐child transmission of HIV and syphilis, Second edition [Internet]. 2017. [cited 2022 Dec 10]. Available from: https://apps.who.int/iris/bitstream/handle/10665/259517/9789241513272‐en...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

