Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer's disease
- PMID: 38528511
- PMCID: PMC10964680
- DOI: 10.1186/s12916-024-03359-2
Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer's disease
Abstract
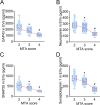
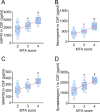
Background: Synaptic dysfunction with reduced synaptic protein levels is a core feature of Alzheimer's disease (AD). Synaptic proteins play a central role in memory processing, learning, and AD pathogenesis. Evidence suggests that synaptic proteins in plasma neuronal-derived extracellular vesicles (EVs) are reduced in patients with AD. However, it remains unclear whether levels of synaptic proteins in EVs are associated with hippocampal atrophy of AD and whether upregulating the expression of these synaptic proteins has a beneficial effect on AD.
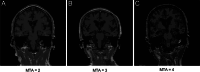
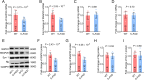
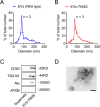
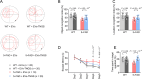
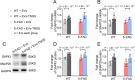
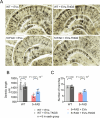
Methods: In this study, we included 57 patients with AD and 56 healthy controls. We evaluated their brain atrophy through magnetic resonance imaging using the medial temporal lobe atrophy score. We measured the levels of four synaptic proteins, including synaptosome-associated protein 25 (SNAP25), growth-associated protein 43 (GAP43), neurogranin, and synaptotagmin 1 in both plasma neuronal-derived EVs and cerebrospinal fluid (CSF). We further examined the association of synaptic protein levels with brain atrophy. We also evaluated the levels of these synaptic proteins in the brains of 5×FAD mice. Then, we loaded rabies virus glycoprotein-engineered EVs with messenger RNAs (mRNAs) encoding GAP43 and SNAP25 and administered these EVs to 5×FAD mice. After treatment, synaptic proteins, dendritic density, and cognitive function were evaluated.
Results: The results showed that GAP43, SNAP25, neurogranin, and synaptotagmin 1 were decreased in neuronal-derived EVs but increased in CSF in patients with AD, and the changes corresponded to the severity of brain atrophy. GAP43 and SNAP25 were decreased in the brains of 5×FAD mice. The engineered EVs efficiently and stably delivered these synaptic proteins to the brain, where synaptic protein levels were markedly upregulated. Upregulation of synaptic protein expression could ameliorate cognitive impairment in AD by promoting dendritic density. This marks the first successful delivery of synaptic protein mRNAs via EVs in AD mice, yielding remarkable therapeutic effects.
Conclusions: Synaptic proteins are closely related to AD processes. Delivery of synaptic protein mRNAs via EVs stands as a promising effective precision treatment strategy for AD, which significantly advances the current understanding of therapeutic approaches for the disease.
Keywords: Alzheimer’s disease; Extracellular vesicles; Growth-associated protein 43; Messenger RNAs; Synaptic dysfunction; Synaptosome-associated protein 25.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Plasma neuregulin 1 as a synaptic biomarker in Alzheimer's disease: a discovery cohort study.Alzheimers Res Ther. 2022 May 23;14(1):71. doi: 10.1186/s13195-022-01014-7. Alzheimers Res Ther. 2022. PMID: 35606871 Free PMC article.
-
Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage.Alzheimers Dement. 2021 Jan;17(1):49-60. doi: 10.1002/alz.12166. Epub 2020 Aug 10. Alzheimers Dement. 2021. PMID: 32776690 Free PMC article.
-
Intranasal delivery of engineered extracellular vesicles loaded with miR-206-3p antagomir ameliorates Alzheimer's disease phenotypes.Theranostics. 2024 Nov 4;14(19):7623-7644. doi: 10.7150/thno.103596. eCollection 2024. Theranostics. 2024. PMID: 39659569 Free PMC article.
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Synaptic Proteins as Fluid Biomarkers in Alzheimer's Disease: A Systematic Review and Meta-Analysis.J Alzheimers Dis. 2022;90(4):1381-1393. doi: 10.3233/JAD-220515. J Alzheimers Dis. 2022. PMID: 36278349
Cited by
-
Increased Expression of Synaptic Vesicle Glycoprotein 2A (SV2A) in the Brain of Chronic Diabetic Rats.Synapse. 2025 May;79(3):e70018. doi: 10.1002/syn.70018. Synapse. 2025. PMID: 40317510 Free PMC article.
-
An Overview on the Physiopathology of the Blood-Brain Barrier and the Lipid-Based Nanocarriers for Central Nervous System Delivery.Pharmaceutics. 2024 Jun 22;16(7):849. doi: 10.3390/pharmaceutics16070849. Pharmaceutics. 2024. PMID: 39065547 Free PMC article. Review.
-
The neuroimmune nexus: unraveling the role of the mtDNA-cGAS-STING signal pathway in Alzheimer's disease.Mol Neurodegener. 2025 Mar 4;20(1):25. doi: 10.1186/s13024-025-00815-2. Mol Neurodegener. 2025. PMID: 40038765 Free PMC article. Review.
-
Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio).Toxics. 2024 Apr 11;12(4):282. doi: 10.3390/toxics12040282. Toxics. 2024. PMID: 38668505 Free PMC article.
-
Extracellular Vesicles: A Promising Therapeutic Approach to Alzheimer's Disease.Curr Alzheimer Res. 2024;21(9):615-624. doi: 10.2174/0115672050365314250112042136. Curr Alzheimer Res. 2024. PMID: 39878107 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

