This is a preprint.
HB-EGF and EGF infusion following CNS demyelination mitigates age-related decline in regeneration of oligodendrocytes from neural precursor cells originating in the ventricular-subventricular zone
- PMID: 38529498
- PMCID: PMC10962700
- DOI: 10.1101/2024.02.26.582092
HB-EGF and EGF infusion following CNS demyelination mitigates age-related decline in regeneration of oligodendrocytes from neural precursor cells originating in the ventricular-subventricular zone
Abstract
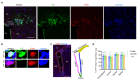
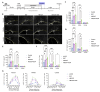
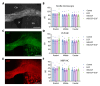
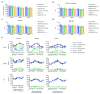
In multiple sclerosis (MS), chronic demyelination initiated by immune-mediated destruction of myelin, leads to axonal damage and neuronal cell death, resulting in a progressive decline in neurological function. The development of interventions that potentiate remyelination could hold promise as a novel treatment strategy for MS. To this end, our group has demonstrated that neural precursor cells (NPCs) residing in the ventricular-subventricular zone (V-SVZ) of the adult mouse brain contribute significantly to remyelination in response to central nervous system (CNS) demyelination and can regenerate myelin of normal thickness. However, aging takes its toll on the regenerative potential of NPCs and reduces their contribution to remyelination. In this study, we investigated how aging influences the contribution of NPCs to oligodendrogenesis during the remyelination process and whether the delivery of growth factors into the brains of aged mice could potentiate the oligodendrogenic potential of NPCs. To enable us to map the fate of NPCs in response to demyelination induced at different postnatal ages, Nestin-CreERT2;Rosa26-LSL-eYFP mice were gavaged with tamoxifen at either 8 weeks, 30 weeks or one year of age before being challenged with cuprizone for a period of six weeks. Using osmotic minipumps, we infused heparin-binding EGF-like growth factor (HB-EGF), and/or epidermal growth factor (EGF) into the cisterna magna for a period of two weeks beginning at the peak of cuprizone-induced demyelination (n=6-8 mice per group). Control mice received artificial cerebrospinal fluid (vehicle) alone. Mice were perfused six weeks after cuprizone withdrawal and the contribution of NPCs to oligodendrocyte regeneration in the corpus callosum was assessed. Our data reveal that although NPC-derived oligodendrocyte generation declined dramatically with age, this decline was partially reversed by growth factor infusion. Notably, co-infusion of EGF and HB-EGF increased oligodendrocyte regeneration twofold in some regions of the corpus callosum. Our results shed light on the beneficial effects of EGF and HB-EGF for increasing the contribution of NPCs to remyelination and indicate their therapeutic potential to combat the negative effects of aging upon remyelination efficacy.
Conflict of interest statement
Conflict of Interest: The authors declare no competing financial interests.
Figures





Similar articles
-
Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination.J Neurosci. 2014 Oct 15;34(42):14128-46. doi: 10.1523/JNEUROSCI.3491-13.2014. J Neurosci. 2014. PMID: 25319708 Free PMC article.
-
Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination.J Neurosci. 2019 Jul 10;39(28):5481-5492. doi: 10.1523/JNEUROSCI.0227-18.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138656 Free PMC article.
-
Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum.Neuroimage. 2014 Feb 1;86:99-110. doi: 10.1016/j.neuroimage.2013.07.080. Epub 2013 Aug 7. Neuroimage. 2014. PMID: 23933305
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
References
-
- Zawadzka M., Rivers L.E., Fancy S.P., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M., et al. (2010). CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578–590. 10.1016/j.stem.2010.04.002. - DOI - PMC - PubMed
-
- Xing Y.L., Roth P.T., Stratton J.A., Chuang B.H., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., and Merson T.D. (2014). Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci 34, 14128–14146. 10.1523/JNEUROSCI.3491-13.2014. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
