Altered PBP4 and GdpP functions synergistically mediate MRSA-like high-level, broad-spectrum β-lactam resistance in Staphylococcus aureus
- PMID: 38530033
- PMCID: PMC11077961
- DOI: 10.1128/mbio.02889-23
Altered PBP4 and GdpP functions synergistically mediate MRSA-like high-level, broad-spectrum β-lactam resistance in Staphylococcus aureus
Abstract
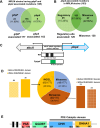
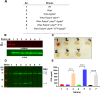
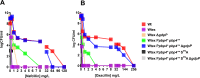
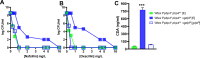
Infections caused by Staphylococcus aureus are a leading cause of mortality worldwide. S. aureus infections caused by methicillin-resistant Staphylococcus aureus (MRSA) are particularly difficult to treat due to their resistance to next-generation β-lactams (NGBs) such as methicillin, nafcillin, and oxacillin. Resistance to NGBs, which is alternatively known as broad-spectrum β-lactam resistance, is classically mediated by PBP2a, a penicillin-binding protein encoded by mecA (or mecC) in MRSA. Thus, presence of mec genes among S. aureus spp. serves as the predictor of resistance to NGBs and facilitates determination of the proper therapeutic strategy for a staphylococcal infection. Although far less appreciated, mecA-deficient S. aureus strains can also exhibit NGB resistance. These strains, which are collectively termed as methicillin-resistant lacking mec (MRLM), are currently being identified in increasing numbers among natural resistant isolates of S. aureus. The mechanism/s through which MRLMs produce resistance to NGBs remains unknown. In this study, we demonstrate that mutations that alter PBP4 and GdpP functions, which are often present among MRLMs, can synergistically mediate resistance to NGBs. Furthermore, our results unravel that this novel mechanism potentially enables MRLMs to produce resistance toward NGBs at levels comparable to those of MRSAs. Our study provides a fresh new perspective about alternative mechanisms of NGB resistance, challenging our current overall understanding of high-level, broad-spectrum β-lactam resistance in S. aureus. It thus suggests reconsideration of the current approach toward diagnosis and treatment of β-lactam-resistant S. aureus infections.
Importance:
In Staphylococcus aureus, high-level, broad-spectrum resistance to β-lactams such as methicillin, also referred to as methicillin resistance, is largely attributed to mecA. This study demonstrates that S. aureus strains that lack mecA but contain mutations that functionally alter PBP4 and GdpP can also mediate high-level, broad-spectrum resistance to β-lactams. Resistance brought about by the synergistic action of functionally altered PBP4 and GdpP was phenotypically comparable to that displayed by mecA, as seen by increased bacterial survival in the presence of β-lactams. An analysis of mutations detected in naturally isolated strains of S. aureus revealed that a significant proportion of them had similar pbp4 and
Keywords: gdpP; methicillin-resistant lacking mec (MRLM); pbp4; β-lactam resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
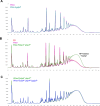
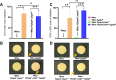
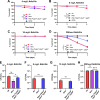
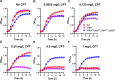
Update of
-
Altered PBP4 and GdpP functions synergistically mediate MRSA-like high-level, broad-spectrum β-lactam resistance in Staphylococcus aureus.bioRxiv [Preprint]. 2023 Oct 27:2023.10.26.564222. doi: 10.1101/2023.10.26.564222. bioRxiv. 2023. Update in: mBio. 2024 May 8;15(5):e0288923. doi: 10.1128/mbio.02889-23. PMID: 37961375 Free PMC article. Updated. Preprint.
Comment in
-
A MRSA mystery: how PBP4 and cyclic-di-AMP join forces against β-lactam antibiotics.mBio. 2024 Aug 14;15(8):e0121024. doi: 10.1128/mbio.01210-24. Epub 2024 Jul 19. mBio. 2024. PMID: 39028200 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
