Vessel and Airway Characteristics in One-Year Computed Tomography-defined Rapid Emphysema Progression: SPIROMICS
- PMID: 38530051
- PMCID: PMC11284327
- DOI: 10.1513/AnnalsATS.202304-383OC
Vessel and Airway Characteristics in One-Year Computed Tomography-defined Rapid Emphysema Progression: SPIROMICS
Abstract
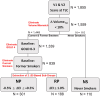
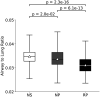
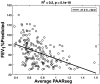
Rationale: Rates of emphysema progression vary in chronic obstructive pulmonary disease (COPD), and the relationships with vascular and airway pathophysiology remain unclear. Objectives: We sought to determine if indices of peripheral (segmental and beyond) pulmonary arterial dilation measured on computed tomography (CT) are associated with a 1-year index of emphysema (EI; percentage of voxels <-950 Hounsfield units) progression. Methods: Five hundred ninety-nine former and never-smokers (Global Initiative for Chronic Obstructive Lung Disease stages 0-3) were evaluated from the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohort: rapid emphysema progressors (RPs; n = 188, 1-year ΔEI > 1%), nonprogressors (n = 301, 1-year ΔEI ± 0.5%), and never-smokers (n = 110). Segmental pulmonary arterial cross-sectional areas were standardized to associated airway luminal areas (segmental pulmonary artery-to-airway ratio [PAARseg]). Full-inspiratory CT scan-derived total (arteries and veins) pulmonary vascular volume (TPVV) was compared with small vessel volume (radius smaller than 0.75 mm). Ratios of airway to lung volume (an index of dysanapsis and COPD risk) were compared with ratios of TPVV to lung volume. Results: Compared with nonprogressors, RPs exhibited significantly larger PAARseg (0.73 ± 0.29 vs. 0.67 ± 0.23; P = 0.001), lower ratios of TPVV to lung volume (3.21 ± 0.42% vs. 3.48 ± 0.38%; P = 5.0 × 10-12), lower ratios of airway to lung volume (0.031 ± 0.003 vs. 0.034 ± 0.004; P = 6.1 × 10-13), and larger ratios of small vessel volume to TPVV (37.91 ± 4.26% vs. 35.53 ± 4.89%; P = 1.9 × 10-7). In adjusted analyses, an increment of 1 standard deviation in PAARseg was associated with a 98.4% higher rate of severe exacerbations (95% confidence interval, 29-206%; P = 0.002) and 79.3% higher odds of being in the RP group (95% confidence interval, 24-157%; P = 0.001). At 2-year follow-up, the CT-defined RP group demonstrated a significant decline in postbronchodilator percentage predicted forced expiratory volume in 1 second. Conclusions: Rapid one-year progression of emphysema was associated with indices indicative of higher peripheral pulmonary vascular resistance and a possible role played by pulmonary vascular-airway dysanapsis.
Keywords: chronic obstructive pulmonary disease; lung imaging; multicenter trials; pulmonary parenchyma; quantitative CT.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

