Concurrent Atezolizumab Plus Bevacizumab and High-Dose External Beam Radiotherapy for Highly Advanced Hepatocellular Carcinoma
- PMID: 38530254
- PMCID: PMC11224977
- DOI: 10.1093/oncolo/oyae048
Concurrent Atezolizumab Plus Bevacizumab and High-Dose External Beam Radiotherapy for Highly Advanced Hepatocellular Carcinoma
Abstract
Background: Atezolizumab plus bevacizumab (atezo-bev) has been recommended for advanced hepatocellular carcinoma (HCC). High-dose external beam radiotherapy (RT) is recognized for its excellent local tumor control. The efficacy and safety of concurrent atezo-bev with RT for highly advanced HCC has been minimally explored.
Methods: In this preliminary retrospective study, we assessed patients with highly advanced HCC, characterized by Vp4 portal vein thrombosis or tumors exceeding 50% of liver volume, who received concurrent atezo-bev and RT (group A). Group A included 13 patients who received proton radiation at a dose of 72.6 GyE in 22 fractions, and one patient who received photon radiation at a dose of 54 Gy in 18 fractions. This group was compared with 34 similar patients treated atezo-bev alone as a control (group B). The primary objectives were to evaluate the objective response rate (ORR), overall survival (OS), and safety.
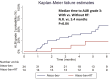
Results: Baseline characteristics were similar between groups, except for a higher incidence of Vp4 portal vein thrombosis in group A (78.6% vs. 21.4%, P = .05). Group A achieved a higher ORR (50.0% vs. 11.8%, P < .01) and a longer OS (not reached vs. 5.5 months, P = .01) after a median follow-up of 5.2 months. Multivariate analysis indicated that concurrent RT independently favored longer OS (hazard ratio: 0.18; 95% CI, 0.05-0.63, P < .01). Group A did not increase any grade adverse events (78.6% vs. 58.8%, P = .19) or severe adverse events of grade ≥ 3 (14.3% vs. 14.7%, P = .97) compared to group B.
Conclusions: The concurrent high-dose external beam radiotherapy appears to safely enhance the effectiveness of atezolizumab plus bevacizumab for highly advanced patients with HCC. Further studies are warranted to confirm these findings.
Keywords: atezolizumab plus bevacizumab; hepatocellular carcinoma; portal vein thrombosis; radiotherapy; toxicity.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors indicated no financial relationships.
Figures


References
-
- Finn RS, Qin S, Ikeda M, et al.. Abstract CT009: IMbrave150: Updated efficacy and safety by risk status in patients (pts) receiving atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment for unresectable hepatocellular carcinoma (HCC). Cancer Res. 2021;81(13_Supplement):CT009-CT009. 10.1158/1538-7445.am2021-ct009 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

