Stratifying Disease Progression in Patients With Cardiac ATTR Amyloidosis
- PMID: 38530684
- PMCID: PMC11004588
- DOI: 10.1016/j.jacc.2023.12.036
Stratifying Disease Progression in Patients With Cardiac ATTR Amyloidosis
Abstract
Background: Transthyretin cardiac amyloidosis (ATTR-CA) is a progressive cardiomyopathy. The clinical course varies among individuals and there are no established measures to assess disease progression.
Objectives: The goal of this study was to assess the prognostic importance of an increase in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and outpatient diuretic intensification (ODI) as markers of disease progression in a large cohort of patients with ATTR-CA.
Methods: We evaluated landmark survival analysis based on worsening of NT-proBNP and requirement for ODI between time of diagnosis and a 1-year visit, and subsequent mortality in 2,275 patients with ATTR-CA from 7 specialist centers. The variables were developed in the National Amyloidosis Centre (NAC) cohort (n = 1,598) and validated in the external cohort from the remaining centers (n = 677).
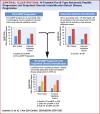
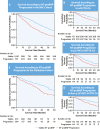
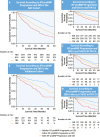
Results: Between baseline and 1-year visits, 551 (34.5%) NAC patients and 204 (30.1%) patients in the external validation cohort experienced NT-proBNP progression (NT-proBNP increase >700 ng/L and >30%), which was associated with mortality (NAC cohort: HR: 1.82; 95% CI: 1.57-2.10; P < 0.001; validation cohort: HR: 1.75; 95% CI: 1.32-2.33; P < 0.001). At 1 year, 451 (28.2%) NAC patients and 301 (44.5%) patients in the external validation cohort experienced ODI, which was associated with mortality (NAC cohort: HR: 1.88; 95% CI: 1.62-2.18; P < 0.001; validation cohort: HR: 2.05; 95% CI: 1.53-2.74; P < 0.001). When compared with patients with a stable NT-proBNP and stable diuretic dose, a higher risk of mortality was observed in those experiencing either NT-proBNP progression or ODI (NAC cohort: HR: 1.93; 95% CI: 1.65-2.27; P < 0.001; validation cohort: HR: 1.94; 95% CI: 1.36-2.77; P < 0.001), and those experiencing both NT-proBNP progression and ODI (NAC cohort: HR: 2.98; 95% CI: 2.42-3.67; P < 0.001; validation cohort: HR: 3.23; 95% CI: 2.17-4.79; P < 0.001).
Conclusions: NT-proBNP progression and ODI are frequent and consistently associated with an increased risk of mortality. Combining both variables produces a simple, universally applicable model that detects disease progression in ATTR-CA.
Keywords: NT-proBNP; cardiac ATTR amyloidosis; disease progression; outpatient diuretic intensification.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Fontana is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447); and has received consulting income from Intellia, Novo Nordisk, Pfizer, Eidos, Prothena, Akcea, Alnylam, Caleum, Alexion, Janssen, Ionis, and AstraZeneca. Dr Gilmore has received consulting income from Ionis, Eidos, Intellia, Alnylam, and Pfizer. Dr Wechalekar has received consulting income from Alexia, AstraZeneca, Janssen, Attralus, and Prothena. Dr Cappelli has received consulting income from Alnylam, Pfizer, AstraZeneca, Bridgebio, and Novo Nordisk. Dr Cipriani has received consulting income from AstraZeneca. Dr Solomon has received research grants from Alexion, Alnylam, AstraZeneca, Bellerophon, Bayer, BMS, Cytokinetics, Eidos, Gossamer, GSK, Ionis, Lilly, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and has served as a consultant for Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, and Valo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Ioannou A., Patel R.K., Razvi Y., et al. Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. J Am Coll Cardiol Img. 2022;14:464–477.
-
- Porcari A., Razvi Y., Masi A., et al. Prevalence, characteristics and outcomes of older patients with hereditary versus wild-type transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2023;25:515–524. - PubMed
-
- Maurer M.S., Schwartz J.H., Gundapaneni B., et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

