Effects of Tirzepatide Versus Basal Insulins in People With Type 2 Diabetes and Different Baseline Glycemic Patterns: Post Hoc Analyses of the SURPASS-3 and SURPASS-4 Trials
- PMID: 38530948
- PMCID: PMC11116914
- DOI: 10.2337/dc23-2366
Effects of Tirzepatide Versus Basal Insulins in People With Type 2 Diabetes and Different Baseline Glycemic Patterns: Post Hoc Analyses of the SURPASS-3 and SURPASS-4 Trials
Abstract
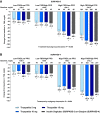
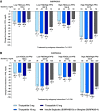
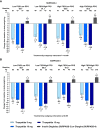
Objective: This post hoc analysis assessed change from baseline to week 52 in glycemic parameters for tirzepatide (5, 10, 15 mg) versus insulin degludec (SURPASS-3 trial) and glargine (SURPASS-4 trial) in people with type 2 diabetes and different baseline glycemic patterns, based on fasting serum glucose (FSG) and postprandial glucose (PPG) values.
Research design and methods: Participant subgroups with low FSG/low PPG, low FSG/high PPG, high FSG/low PPG, and high FSG/high PPG were defined according to the median values of these measures.
Results: All tirzepatide doses and basal insulins were associated with decreased HbA1c, FSG, and PPG values from baseline to week 52 in all subgroups (P < 0.05). Within each subgroup, HbA1c and PPG decreases were greater with tirzepatide than insulin (P < 0.05). FSG decreases were generally similar. There were no differential treatment effects by FSG/PPG subgroup.
Conclusions: In this post hoc analysis, tirzepatide was associated with superior glycemic control compared with insulin, irrespective of baseline glycemic pattern.
© 2024 by the American Diabetes Association.
Figures


References
-
- Leiter LA, Ceriello A, Davidson JA, et al. .; International Prandial Glucose Regulation Study Group . Postprandial glucose regulation: new data and new implications. Clin Ther 2005;27(Suppl. B):S42–S56 - PubMed
-
- Woerle HJ, Neumann C, Zschau S, et al. . Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes. Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–285 - PubMed
-
- Riddle MC. The current schemes of insulin therapy: pro and contra. Diabetes Res Clin Pract 2021;175:108817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

