Randomized, Double-Blind Phase III Study of Pazopanib Versus Placebo in Patients With Metastatic Renal Cell Carcinoma Who Have No Evidence of Disease After Metastasectomy: ECOG-ACRIN E2810
- PMID: 38531002
- PMCID: PMC11514296
- DOI: 10.1200/JCO.23.01544
Randomized, Double-Blind Phase III Study of Pazopanib Versus Placebo in Patients With Metastatic Renal Cell Carcinoma Who Have No Evidence of Disease After Metastasectomy: ECOG-ACRIN E2810
Abstract
Purpose: Patients with no evidence of disease (NED) after metastasectomy for renal cell carcinoma are at high risk of recurrence. Pazopanib is an inhibitor of vascular endothelial growth factor receptor and other kinases that improves progression-free survival in patients with metastatic RCC (mRCC). We conducted a randomized, double-blind, placebo-controlled multicenter study to test whether pazopanib would improve disease-free survival (DFS) in patients with mRCC rendered NED after metastasectomy.
Patients and methods: Patients with NED after metastasectomy were randomly assigned 1:1 to receive pazopanib 800 mg once daily versus placebo for 52 weeks. The study was designed to observe an improvement in DFS from 25% to 45% with pazopanib at 3 years, corresponding to 42% reduction in the DFS event rate.
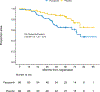
Results: From August 2012 to July 2017, 129 patients were enrolled. The study was unblinded after 83 DFS events (92% information). The study did not meet its primary end point. An updated analysis at 60.5-month median follow-up from random assignment (95% CI, 59.3 to 71.0) showed that the 3-year DFS was 27.4% (95% CI, 17.9 to 41.7) for pazopanib and 21.9% (95% CI, 13.3 to 36.2) for placebo. Hazard ratio (HR) for DFS was 0.90 ([95% CI, 0.60 to 1.34]; Pone-sided = .29) in favor of pazopanib. Three-year overall survival (OS) was 81.9% (95% CI, 72.7 to 92.2) for pazopanib and 91.4% (95% CI, 84.4 to 98.9) for placebo. The HR for OS was 2.55 (95% CI, 1.23 to 5.27) in favor of placebo (Ptwo-sided = .012). Health-related quality-of-life measures deteriorated in the pazopanib group during the treatment period.
Conclusion: Pazopanib did not improve DFS as the primary end point compared with blinded placebo in patients with mRCC with NED after metastasectomy. In addition, there was a concerning trend favoring placebo in OS.
Trial registration: ClinicalTrials.gov NCT01575548.
Figures
References
-
- Tran J, Ornstein MC: Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncology Practice 18:187–196, 2022 - PubMed
-
- Barney JD, Churchill EJ: Adenocarcinoma of the Kidney with Metastasis to the Lung: Cured by Nephrectomy and Lobectomy. Journal of Urology 42:269–276, 1939
-
- Kavolius JP, Mastorakos DP, Pavlovich C, et al.: Resection of metastatic renal cell carcinoma. J Clin Oncol 16:2261–6, 1998 - PubMed
-
- Alt AL, Boorjian SA, Lohse CM, et al.: Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 117:2873–82, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233178/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA239767/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233247/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical