Real-World Effectiveness of Ustekinumab in Ulcerative Colitis in a United States Multicenter Cohort Consortium
- PMID: 38531068
- PMCID: PMC11700893
- DOI: 10.1093/ibd/izae058
Real-World Effectiveness of Ustekinumab in Ulcerative Colitis in a United States Multicenter Cohort Consortium
Abstract
Background: Pivotal trials have shown that ustekinumab is effective in ulcerative colitis (UC). However, the population included in these trials do not represent the cohort of patients treated in the real world. In this study, we aimed to describe the effectiveness and safety of ustekinumab in a clinical cohort of patients with UC.
Methods: We performed a multicenter retrospective cohort study and included patients with active UC starting ustekinumab. Variables collected included demographics, clinical data, and disease activity (measured using partial Mayo score [PMS] and endoscopic Mayo score) at follow-up. The primary outcomes were cumulative rates of steroid-free clinical and biochemical remission (SFCBR), defined as a PMS <2 while off steroids and a normal C-reactive protein and/or fecal calprotectin.
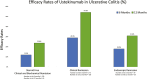
Results: A total of 245 patients met inclusion criteria. The median time of follow-up was 33 (interquartile range, 17-53) weeks, and 214 (87.3%) had previous exposure to a biologic and/or tofacitinib. Rates of SFCBR, clinical remission, and endoscopic remission at 6 and 12 months were 12.0% (n = 16 of 139), 29.0% (n = 71 of 175), and 18.0% (n = 7 of 39), and 23.8% (n = 15 of 63), 54.3% (n = 57 of 105), and 31.0% (n = 9 of 29), respectively. Non-Hispanic White race, higher baseline PMS, and the use of concomitant corticosteroids were independently associated with failure to achieve SFCBR. Of the 73 that were dose escalated, 28.4% did not respond, 49.3% experienced a benefit, and 21.6% achieved remission.
Conclusions: In a population enriched with refractory UC, ustekinumab was well tolerated and induced remission in a significant number of patients. Larger studies with a longer follow-up are warranted.
Keywords: colectomy; dose escalation; efficacy; ulcerative colitis; ustekinumab.
Plain language summary
Ustekinumab was shown to be efficacious and safe in a population of patients with refractory ulcerative colitis. Those patients with exposure to multiple drug classes and higher disease burden at baseline are less likely to respond.
© The Author(s) 2024. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
A.K.Y. has served on the advisory board and/or as a consultant for Takeda, Pfizer, Arena, AbbVie, Bristol Myers Squibb, and Celltrion. A.P. has served as a consultant for AbbVie; and as a speaker for Bristol Myers Squibb, Takeda, Eli Lilly, Janssen, and Pfizer. B.L.C. has served on the advisory board and as a consultant for AbbVie and TARGET RWE; served as a speaker for AbbVie; and received educational grant support from Pfizer. E.L. has served as a consultant for AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iota Biosciences, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Protagonist, Sun, Surrozen, Takeda, and UCB, has received research support from AbbVie, AstraZeneca, Bristol-Myers Squibb, Celgene/Receptos, Genentech, Gilead, Gossamer Bio, Janssen, Takeda, Theravance, and UCB; and is shareowner of Exact Sciences. P.D. has served as a consultant or on the advisory board for Janssen, Pfizer, Prometheus Biosciences, Boehringer Ingelheim, AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, CorEvitas LLC, and Scipher Medicine Corporation; and received funding under a sponsored research agreement unrelated to the data in the article from Takeda Pharmaceutical, Arena Pharmaceuticals, Bristol Myers Squibb-Celgene, and Boehringer Ingelheim. J.P. has received grants from Takeda and AbbVie; served as a consultant for Verastem; and served on the advisory board for Pfizer and Janssen. R.U. has served as a consultant for AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Lilly, Pfizer, Roivant, and Takeda. G.S. has received grants from Pfizer.
Figures


References
-
- Sands BE, Sandborn WJ, Panaccione R, et al.; UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201-1214. - PubMed
-
- Ha C, Ullman TA, Siegel CA, et al.Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002-1007, quiz e78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

