Computationally guided AAV engineering for enhanced gene delivery
- PMID: 38531696
- PMCID: PMC11456259
- DOI: 10.1016/j.tibs.2024.03.002
Computationally guided AAV engineering for enhanced gene delivery
Abstract
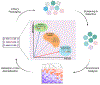
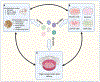
Gene delivery vehicles based on adeno-associated viruses (AAVs) are enabling increasing success in human clinical trials, and they offer the promise of treating a broad spectrum of both genetic and non-genetic disorders. However, delivery efficiency and targeting must be improved to enable safe and effective therapies. In recent years, considerable effort has been invested in creating AAV variants with improved delivery, and computational approaches have been increasingly harnessed for AAV engineering. In this review, we discuss how computationally designed AAV libraries are enabling directed evolution. Specifically, we highlight approaches that harness sequences outputted by next-generation sequencing (NGS) coupled with machine learning (ML) to generate new functional AAV capsids and related regulatory elements, pushing the frontier of what vector engineering and gene therapy may achieve.
Keywords: AAV libraries; ancestral sequence reconstruction; directed evolution; machine learning; next-generation sequencing; protein engineering.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests D.V.S. and J.L. are inventors on patents related to viral vector-directed evolution and engineered AAV variants.
Figures



References
-
- Zhu D. et al. (2021) Adeno-Associated Virus Vector for Central Nervous System Gene Therapy. Trends Mol Med 27, 524–537 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

