How comparable are patient outcomes in the "real-world" with populations studied in pivotal AML trials?
- PMID: 38531863
- PMCID: PMC10965987
- DOI: 10.1038/s41408-024-00996-x
How comparable are patient outcomes in the "real-world" with populations studied in pivotal AML trials?
Abstract
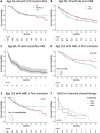
Despite an increasing desire to use historical cohorts as "synthetic" controls for new drug evaluation, limited data exist regarding the comparability of real-world outcomes to those in clinical trials. Governmental cancer data often lacks details on treatment, response, and molecular characterization of disease sub-groups. The Australasian Leukaemia and Lymphoma Group National Blood Cancer Registry (ALLG NBCR) includes source information on morphology, cytogenetics, flow cytometry, and molecular features linked to treatment received (including transplantation), response to treatment, relapse, and survival outcome. Using data from 942 AML patients enrolled between 2012-2018, we assessed age and disease-matched control and interventional populations from published randomized trials that led to the registration of midostaurin, gemtuzumab ozogamicin, CPX-351, oral azacitidine, and venetoclax. Our analyses highlight important differences in real-world outcomes compared to clinical trial populations, including variations in anthracycline type, cytarabine intensity and scheduling during consolidation, and the frequency of allogeneic hematopoietic cell transplantation in first remission. Although real-world outcomes were comparable to some published studies, notable differences were apparent in others. If historical datasets were used to assess the impact of novel therapies, this work underscores the need to assess diverse datasets to enable geographic differences in treatment outcomes to be accounted for.
© 2024. The Author(s).
Conflict of interest statement
AB: Advisory role with honoraria for AbbVie, Amgen, Astellas, Pfizer, Novartis, Jazz, Takeda, Gilead-Kite, SentiBio; speaker fees from BMS, Amgen. AWR: Clinical research funding to Institution from AbbVie and Amgen. Inventor on a patent related to venetoclax, and receives a fraction of royalty payments to the Walter and Eliza Hall Institute of Medical Research related to venetoclax. CC: Advisory role with honoraria for AbbVie, Pfizer, Sumitomo Pharma. PM: Advisory Board and/or speaker for AbbVie, AstraZeneca, Astellas, Beigene, Gilead, Janssen, Minarine, MSD, Novartis, Otsuka, Pfizer, Roche. CYF: has received honoraria from AbbVie, Amgen, Beigene, Bristol-Myers Squibb, Jazz, Novartis, Pfizer and Servier; has acted as a consultant/advisor for AbbVie, Amgen, Astellas Pharma, Bristol-Myers Squibb, Jazz, Novartis, Pfizer and Servier; and has received research funding from Amgen, Astellas and Jazz. DP: Honoraria from Bristol Meyers Squibb, Jazz Pharmaceuticals, Link, Bastion Education, Gilead. All honoraria paid to institution only. MH: Advisory role with honoraria for Gilead, Roche, Otsuka, Takeda, Janssen, Beigene. JS: Advisory role with honoraria for Astellas, BMS, Mundipharma, Novartis, Otsuka, Pfizer; research funding to institution from Amgen, Astex, BMS; speakers fees from Mundipharma, Novartis. AW: Advisory role with honoraria for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Roche, BMS, Celgene, Gilead, Pfizer, Janssen, Jazz, Novartis, Servier; clinical research funding to Institution from AbbVie, Servier, Celgene-BMS, Astra Zeneca, Amgen, Novartis; receives a fraction of royalty payments to the Walter and Eliza Hall Institute of Medical Research related to venetoclax.
Figures
References
-
- Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie J-N, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16. doi: 10.1016/S0140-6736(12)60485-1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

