Rapid development of double-hit mRNA antibody cocktail against orthopoxviruses
- PMID: 38531869
- PMCID: PMC10966106
- DOI: 10.1038/s41392-024-01766-8
Rapid development of double-hit mRNA antibody cocktail against orthopoxviruses
Abstract
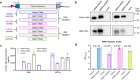
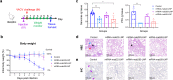
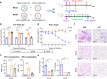
The Orthopoxvirus genus, especially variola virus (VARV), monkeypox virus (MPXV), remains a significant public health threat worldwide. The development of therapeutic antibodies against orthopoxviruses is largely hampered by the high cost of antibody engineering and manufacturing processes. mRNA-encoded antibodies have emerged as a powerful and universal platform for rapid antibody production. Herein, by using the established lipid nanoparticle (LNP)-encapsulated mRNA platform, we constructed four mRNA combinations that encode monoclonal antibodies with broad neutralization activities against orthopoxviruses. In vivo characterization demonstrated that a single intravenous injection of each LNP-encapsulated mRNA antibody in mice resulted in the rapid production of neutralizing antibodies. More importantly, mRNA antibody treatments showed significant protection from weight loss and mortality in the vaccinia virus (VACV) lethal challenge mouse model, and a unique mRNA antibody cocktail, Mix2a, exhibited superior in vivo protection by targeting both intracellular mature virus (IMV)-form and extracellular enveloped virus (EEV)-form viruses. In summary, our results demonstrate the proof-of-concept production of orthopoxvirus antibodies via the LNP-mRNA platform, highlighting the great potential of tailored mRNA antibody combinations as a universal strategy to combat orthopoxvirus as well as other emerging viruses.
© 2024. The Author(s).
Conflict of interest statement
C.F.Q., Y.Q.D., H.C., S.Q.Z., R.Y.C., and X.X.S. have filed patents related to the finding reported in this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

