Sex dimorphism controls dysbindin-related cognitive dysfunctions in mice and humans with the contribution of COMT
- PMID: 38532008
- PMCID: PMC11420087
- DOI: 10.1038/s41380-024-02527-3
Sex dimorphism controls dysbindin-related cognitive dysfunctions in mice and humans with the contribution of COMT
Abstract
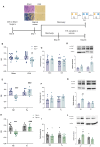
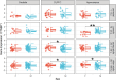
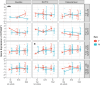
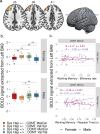
Cognitive dysfunctions are core-enduring symptoms of schizophrenia, with important sex-related differences. Genetic variants of the DTBPN1 gene associated with reduced dysbindin-1 protein (Dys) expression negatively impact cognitive functions in schizophrenia through a functional epistatic interaction with Catechol-O-methyltransferase (COMT). Dys is involved in the trafficking of dopaminergic receptors, crucial for prefrontal cortex (PFC) signaling regulation. Moreover, dopamine signaling is modulated by estrogens via inhibition of COMT expression. We hypothesized a sex dimorphism in Dys-related cognitive functions dependent on COMT and estrogen levels. Our multidisciplinary approach combined behavioral-molecular findings on genetically modified mice, human postmortem Dys expression data, and in vivo fMRI during a working memory task performance. We found cognitive impairments in male mice related to genetic variants characterized by reduced Dys protein expression (pBonferroni = 0.0001), as well as in male humans through a COMT/Dys functional epistatic interaction involving PFC brain activity during working memory (t(23) = -3.21; pFDR = 0.004). Dorsolateral PFC activity was associated with lower working memory performance in males only (p = 0.04). Also, male humans showed decreased Dys expression in dorsolateral PFC during adulthood (pFDR = 0.05). Female Dys mice showed preserved cognitive performances with deficits only with a lack of estrogen tested in an ovariectomy model (pBonferroni = 0.0001), suggesting that genetic variants reducing Dys protein expression could probably become functional in females when the protective effect of estrogens is attenuated, i.e., during menopause. Overall, our results show the differential impact of functional variants of the DTBPN1 gene interacting with COMT on cognitive functions across sexes in mice and humans, underlying the importance of considering sex as a target for patient stratification and precision medicine in schizophrenia.
© 2024. The Author(s).
Conflict of interest statement
AB received consulting fees from Biogen and lecture fees from Otsuka, Janssen, and Lundbeck. GB and GP received lecture fees from Lundbeck. AR received travel fees from Lundbeck. All other authors have no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Riecher-Rossler A, Butler S, Kulkarni J. Sex and gender differences in schizophrenic psychoses-a critical review. Arch Women’s Ment Health. 2018;21:627–48. - PubMed
-
- NIH Inclusion Outreach Toolkit: How to Engage, Recruit, and Retain Women in Clinical Research. https://orwh.od.nih.gov/toolkit/nih-policies-inclusion/guidelines, Accessed Date Accessed 2015 Accessed. (2015).
-
- Hafner H, Maurer K, Loffler W, Riecher-Rossler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. - PubMed
-
- Riecher-Rossler A, Hafner H, Stumbaum M, Maurer K, Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophr Bull. 1994;20:203–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

