Active forgetting and neuropsychiatric diseases
- PMID: 38532011
- PMCID: PMC11420092
- DOI: 10.1038/s41380-024-02521-9
Active forgetting and neuropsychiatric diseases
Abstract
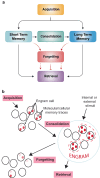
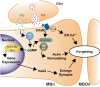
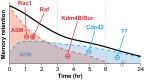
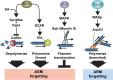
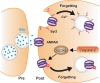
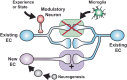
Recent and pioneering animal research has revealed the brain utilizes a variety of molecular, cellular, and network-level mechanisms used to forget memories in a process referred to as "active forgetting". Active forgetting increases behavioral flexibility and removes irrelevant information. Individuals with impaired active forgetting mechanisms can experience intrusive memories, distressing thoughts, and unwanted impulses that occur in neuropsychiatric diseases. The current evidence indicates that active forgetting mechanisms degrade, or mask, molecular and cellular memory traces created in synaptic connections of "engram cells" that are specific for a given memory. Combined molecular genetic/behavioral studies using Drosophila have uncovered a complex system of cellular active-forgetting pathways within engram cells that is regulated by dopamine neurons and involves dopamine-nitric oxide co-transmission and reception, endoplasmic reticulum Ca2+ signaling, and cytoskeletal remodeling machinery regulated by small GTPases. Some of these molecular cellular mechanisms have already been found to be conserved in mammals. Interestingly, some pathways independently regulate forgetting of distinct memory types and temporal phases, suggesting a multi-layering organization of forgetting systems. In mammals, active forgetting also involves modulation of memory trace synaptic strength by altering AMPA receptor trafficking. Furthermore, active-forgetting employs network level mechanisms wherein non-engram neurons, newly born-engram neurons, and glial cells regulate engram synapses in a state and experience dependent manner. Remarkably, there is evidence for potential coordination between the network and cellular level forgetting mechanisms. Finally, subjects with several neuropsychiatric diseases have been tested and shown to be impaired in active forgetting. Insights obtained from research on active forgetting in animal models will continue to enrich our understanding of the brain dysfunctions that occur in neuropsychiatric diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Biology of Forgetting-A Perspective.Neuron. 2017 Aug 2;95(3):490-503. doi: 10.1016/j.neuron.2017.05.039. Neuron. 2017. PMID: 28772119 Free PMC article. Review.
-
Active forgetting of olfactory memories in Drosophila.Prog Brain Res. 2014;208:39-62. doi: 10.1016/B978-0-444-63350-7.00002-4. Prog Brain Res. 2014. PMID: 24767478 Review.
-
Natural forgetting reversibly modulates engram expression.Elife. 2024 Nov 5;12:RP92860. doi: 10.7554/eLife.92860. Elife. 2024. PMID: 39499054 Free PMC article.
-
Molecular Underpinnings of Memory Persistence and Forgetting.J Neurochem. 2025 May;169(5):e70089. doi: 10.1111/jnc.70089. J Neurochem. 2025. PMID: 40411122 Review.
-
Neural, Cellular and Molecular Mechanisms of Active Forgetting.Front Syst Neurosci. 2018 Feb 6;12:3. doi: 10.3389/fnsys.2018.00003. eCollection 2018. Front Syst Neurosci. 2018. PMID: 29467630 Free PMC article. Review.
Cited by
-
Brain mechanisms underlying the inhibitory control of thought.Nat Rev Neurosci. 2025 Jul;26(7):415-437. doi: 10.1038/s41583-025-00929-y. Epub 2025 May 16. Nat Rev Neurosci. 2025. PMID: 40379896 Review.
-
Suprachiasmatic Nucleus Vasoactive Intestinal Peptide Neurons Mediate Light-induced Transient Forgetting.Neurosci Bull. 2025 Jul 16. doi: 10.1007/s12264-025-01456-7. Online ahead of print. Neurosci Bull. 2025. PMID: 40670769
References
-
- Squire LR Memory and Brain. Oxford University Press, 1987.
-
- Dudai Y Memory From A to Z: Keywords, Concepts, and Beyond. Oxford University Press, 2004.
-
- Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

