Macrophage mannose receptor CD206 targeting of fluoride-18 labeled mannosylated dextran: A validation study in mice
- PMID: 38532026
- PMCID: PMC11178572
- DOI: 10.1007/s00259-024-06686-x
Macrophage mannose receptor CD206 targeting of fluoride-18 labeled mannosylated dextran: A validation study in mice
Abstract
Purpose: Aluminum fluoride-18-labeled 1,4,7-triazacyclononane-1,4,7-triacetic acid-conjugated mannosylated dextran derivative (Al[18F]F-NOTA-D10CM) is a new tracer for PET imaging. We report here on in vitro and in vivo validation of the tracer's ability to target the macrophage mannose receptor CD206.
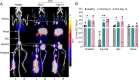
Methods: First, the uptake of intravenously (i.v.) administered Al[18F]F-NOTA-D10CM was compared between wild-type (WT) and CD206-/- knockout (KO) mice. C57BL/6N mice were injected with complete Freund's adjuvant (CFA) in the left hind leg and the uptake of Al[18F]F-NOTA-D10CM after i.v. or intradermal (i.d.) injection was studied at 5 and 14 days after CFA induction of inflammation. Healthy C57BL/6N mice were studied as controls. Mice underwent PET/CT on consecutive days with [18F]FDG, i.v. Al[18F]F-NOTA-D10CM, and i.d. Al[18F]F-NOTA-D10CM. After the last imaging, Al[18F]F-NOTA-D10CM was i.v. injected for an ex vivo biodistribution study and autoradiography of inflamed tissues. Blood plasma samples were analyzed using high-performance liquid chromatography. To evaluate the specificity of Al[18F]F-NOTA-D10CM binding, an in vitro competitive displacement study was performed on inflamed tissue sections using autoradiography. CD206 expression was assessed by immunohistochemical staining.
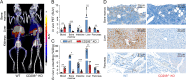
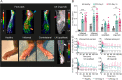
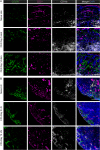
Results: Compared with WT mice, the uptake of Al[18F]F-NOTA-D10CM was significantly lower in several CD206-/- KO mice tissues, including liver (SUV 8.21 ± 2.51 vs. 1.06 ± 0.16, P < 0.001) and bone marrow (SUV 1.63 ± 0.37 vs. 0.22 ± 0.05, P < 0.0001). The uptake of i.v. injected Al[18F]F-NOTA-D10CM was significantly higher in inflamed ankle joint (SUV 0.48 ± 0.13 vs. 0.18 ± 0.05, P < 0.0001) and inflamed foot pad skin (SUV 0.41 ± 0.10 vs. 0.04 ± 0.01, P < 0.0001) than in the corresponding tissues in healthy mice. The i.d.-injected Al[18F]F-NOTA-D10CM revealed differences between CFA-induced lymph node activation and lymph nodes in healthy mice. Ex vivo γ-counting, autoradiography, and immunohistochemistry supported the results, and a decrease of ~ 80% in the binding of Al[18F]F-NOTA-D10CM in the displacement study with excess NOTA-D10CM confirmed that tracer binding was specific. At 60 min after i.v. injection, an average 96.70% of plasma radioactivity was derived from intact Al[18F]F-NOTA-D10CM, indicating good in vivo stability. The uptake of Al[18F]F-NOTA-D10CM into inflamed tissues was positively associated with the area percentage of CD206-positive staining.
Conclusion: The uptake of mannosylated dextran derivative Al[18F]F-NOTA-D10CM correlated with CD206 expression and the tracer appears promising for inflammation imaging.
Keywords: Fluorine-18; Inflammation; Macrophage mannose receptor CD206; Mannosylated dextran; PET/CT.
© 2024. The Author(s).
Conflict of interest statement
AS received fees for consultancy or lecturing from Abbott, AstraZeneca, Janssen, Novartis, and Pfizer outside the current study. The remaining authors have no conflicts of interest to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

