A phase 1/2 study of NS-87/CPX-351 (cytarabine and daunorubicin liposome) in Japanese patients with high-risk acute myeloid leukemia
- PMID: 38532078
- PMCID: PMC11136735
- DOI: 10.1007/s12185-024-03733-z
A phase 1/2 study of NS-87/CPX-351 (cytarabine and daunorubicin liposome) in Japanese patients with high-risk acute myeloid leukemia
Abstract
Objectives: NS-87/CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin. NS-87/CPX-351 exerts antileukemic action by maintaining a synergistic molar ratio of cytarabine to daunorubicin of 5:1 within the liposome while in circulation. Patients with high-risk acute myeloid leukemia (AML), which includes therapy-related AML and AML with myelodysplasia-related changes (AML-MRC), have poorer outcomes than those with other AML.
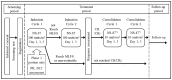
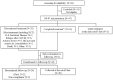
Methodology: This open-label phase 1/2 (P1/2) study was conducted in 47 Japanese patients aged 60-75 years with newly diagnosed high-risk AML to evaluate the pharmacokinetics, safety, and efficacy of NS-87/CPX-351.
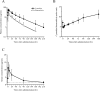
Results: In the 6 patients enrolled in the P1 portion, no dose-limiting toxicities (DLTs) were reported, and 100 units/m2 during the induction cycle was found to be acceptable. Cytarabine and daunorubicin had a long half-life in the terminal phase (32.8 and 28.7 h, respectively). In the 35 patients enrolled in the P2 portion, composite complete remission (CRc; defined as complete remission [CR] or CR with incomplete hematologic recovery [CRi]) was achieved in 60.0% (90% CI: 44.7-74.0) of the patients. Adverse events due to NS-87/CPX-351 were well tolerated.
Outcomes: NS-87/CPX-351 can be considered as a frontline treatment option for Japanese patients with high-risk AML.
Keywords: Acute myeloid leukemia; CPX-351; Cytarabine; Daunorubicin; Liposomal formulation.
© 2024. The Author(s).
Conflict of interest statement
NS-87/CPX-351 was provided by Nippon Shinyaku Co., Ltd. UK reports grants from Astellas Pharma Inc., Abbvie Inc., Bristol-Myers-Squibb K.K., Janssen Pharmaceutical K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Merck & Co., Inc., SymBio Pharmaceuticals Limited., Celgene Corporation., Daiichi Sankyo Company Limited., Sumitomo Pharma Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Mundipharma International., and Takeda Pharmaceutical Company Limited., consulting fees from Astellas Pharma Inc., Amgen Inc., Alnylam Japan K.K., Alexion Pharmaceuticals, Inc., Incyte Corporation., Ono Pharmaceutical Co., Ltd., OHARA Pharmaceutical Co.,Ltd., Kyowa Kirin Co., Sanofi K.K., Sandoz International GmbH, SymBio Pharmaceuticals Limited., Takeda Pharmaceutical Company Limited., Chugai Pharmaceutical Co., Ltd., and Nippon Shinyaku Co., Ltd., and honoraria from Novartis Pharma K.K., Abbvie Inc., Alexion Pharmaceuticals, Inc., Incyte Corporation., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Company Limited., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., and Bristol-Myers-Squibb K.K. AK reports grants from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., Kyowa Kirin Co., Ltd., Nippon Shinyaku Co., Ltd., Eisai Co., Ltd. and Otsuka Pharmaceutical Co., Ltd. YO reports grants from Alexion Pharmaceuticals, Inc. and honoraria from Novartis Pharma K.K. OM reports honoraria from Astellas Pharma Inc., Daiichi Sankyo Company Limited., Otsuka Pharmaceutical Co., Ltd., and Nippon Shinyaku Co., Ltd. YT reports consulting fees and honoraria for lectures and speakers. KH reports grants from FUJIFILM Pharmaceuticals Inc., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Perseus Proteomics Inc., Daiichi Sankyo Company Limited., Abbvie Inc., CURED, Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Zenyaku Holdings Co., Ltd., Nippon Shinyaku Co., Ltd., and Takeda Pharmaceutical Company Limited., Sumitomo Pharma Co., and honoraria from Abbvie Inc., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Novartis Pharma K.K. IT reports honoraria from Asahi Kasei Pharma Corp., Sanofi K.K., Nippon Shinyaku Co., Ltd., Alexion Pharmaceuticals, Inc., and Pfizer Japan Inc., and participation on an advisory board for Chugai Pharmaceutical Co., Ltd., Alexion Pharmaceuticals, Inc., and Asahi Kasei Pharma Corp. AN reports grants from Novartis Pharma K.K. and honoraria from Abbvie Inc. OS reports honoraria from Novartis Pharma K.K., Bristol-Myers-Squibb K.K., Takeda Pharmaceutical Company Limited., AstraZeneca K.K., Janssen Pharmaceutical K.K., Abbvie Inc., and Amgen Inc.
AK reports research funding of the submitted study from Nippon Shinyaku Co., Ltd., grants from Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Asahi Kasei Pharma Corp., Abbvie Inc., Chugai Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Limited., and honoraria from Abbvie Inc., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Pfizer Japan Inc., Bristol-Myers-Squibb K.K., and Janssen Pharmaceutical K.K.
MY reports research funding of the submitted study from Nippon Shinyaku Co., Ltd., honoraria from Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Astellas Pharma Inc., and Abbvie Inc., and leadership in Japan Adult Leukemia Study Group. DA, MK, IS, and KU are employees of Nippon Shinyaku Co., Ltd.
Figures
References
-
- Arber DA, Brunning RD, Le Beau MM, et al. Acute myeloid leukaemia and related precursor neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC Press; 2017. pp. 130–71.
-
- Granfeldt Østgård LS, Medeiros BC, Sengelov H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. doi: 10.1200/JCO.2014.60.0890. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

