Optimized microburst VNS elicits fMRI responses beyond thalamic-specific response from standard VNS
- PMID: 38532258
- PMCID: PMC11093236
- DOI: 10.1002/acn3.52029
Optimized microburst VNS elicits fMRI responses beyond thalamic-specific response from standard VNS
Abstract
Objective: In parallel to standard vagus nerve stimulation (VNS), microburst stimulation delivery has been developed. We evaluated the fMRI-related signal changes associated with standard and optimized microburst stimulation in a proof-of-concept study (NCT03446664).
Methods: Twenty-nine drug-resistant epilepsy patients were prospectively implanted with VNS. Three 3T fMRI scans were collected 2 weeks postimplantation. The maximum tolerated VNS intensity was determined prior to each scan starting at 0.125 mA with 0.125 mA increments. FMRI scans were block-design with alternating 30 sec stimulation [ON] and 30 sec no stimulation [OFF]: Scan 1 utilized standard VNS and Scan 3 optimized microburst parameters to determine target settings. Semi-automated on-site fMRI data processing utilized ON-OFF block modeling to determine VNS-related fMRI activation per stimulation setting. Anatomical thalamic mask was used to derive highest mean thalamic t-value for determination of microburst stimulation parameters. Paired t-tests corrected at P < 0.05 examined differences in fMRI responses to each stimulation type.
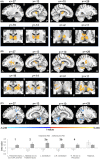
Results: Standard and microburst stimulation intensities at Scans 1 and 3 were similar (P = 0.16). Thalamic fMRI responses were obtained in 28 participants (19 with focal; 9 with generalized seizures). Group activation maps showed standard VNS elicited thalamic activation while optimized microburst VNS showed widespread activation patterns including thalamus. Comparison of stimulation types revealed significantly greater cerebellar, midbrain, and parietal fMRI signal changes in microburst compared to standard VNS. These differences were not associated with seizure responses.
Interpretation: While standard and optimized microburst VNS elicited thalamic activation, microburst also engaged other brain regions. Relationship between these fMRI activation patterns and clinical response warrants further investigation.
Clinical trial registration: The study was registered with clinicaltrials.gov (NCT03446664).
© 2024 LivaNova USA, Inc. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Figures

References
-
- Bolden L, Pati S, Szaflarski J. Neurostimulation, neuromodulation, and the treatment of epilepsies. J Epileptol. 2015;23:45‐59.
-
- Touma L, Dansereau B, Chan AY, et al. Neurostimulation in people with drug‐resistant epilepsy: systematic review and meta‐analysis from the ILAE Surgical Therapies Commission. Epilepsia. 2022;63:1314‐1329. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

