Five-year results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement-the ARISE Study and ARISE Registry Data
- PMID: 38532304
- PMCID: PMC11009017
- DOI: 10.1093/ejcts/ezae121
Five-year results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement-the ARISE Study and ARISE Registry Data
Abstract
Objectives: Decellularized aortic homografts (DAH) were introduced as a new option for aortic valve replacement for young patients.
Methods: A prospective, EU-funded, single-arm, multicentre study in 8 centres evaluating non-cryopreserved DAH for aortic valve replacement.
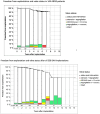
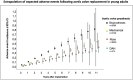
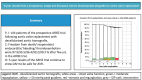
Results: A total of 144 patients (99 male) were prospectively enrolled in the ARISE Trial between October 2015 and October 2018 with a median age of 30.4 years [interquartile range (IQR) 15.9-55.1]; 45% had undergone previous cardiac operations, with 19% having 2 or more previous procedures. The mean implanted DAH diameter was 22.6 mm (standard deviation 2.4). The median operation duration was 312 min (IQR 234-417), the median cardiopulmonary bypass time was 154 min (IQR 118-212) and the median cross-clamp time 121 min (IQR 93-150). No postoperative bypass grafting or renal replacement therapy were required. Two early deaths occurred, 1 due to a LCA thrombus on day 3 and 1 due ventricular arrhythmia 5 h postoperation. There were 3 late deaths, 1 death due to endocarditis 4 months postoperatively and 2 unrelated deaths after 5 and 7 years due to cancer and Morbus Wegener resulting in a total mortality of 3.47%. After a median follow-up of 5.9 years [IQR 5.1-6.4, mean 5.5 years. (standard deviation 1.3) max. 7.6 years], the primary efficacy end-points peak gradient with median 11.0 mmHg (IQR 7.8-17.6) and regurgitation of median 0.5 (IQR 0-0.5) of grade 0-3 were excellent. At 5 years, freedom from death/reoperation/endocarditis/bleeding/thromboembolism were 97.9%/93.5%/96.4%/99.2%/99.3%, respectively.
Conclusions: The 5-year results of the prospective multicentre ARISE trial continue to show DAH to be safe for aortic valve replacement with excellent haemodynamics.
Keywords: Allografts; Aortic valve disease; Decellularization; Tissue engineering.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures

Comment in
-
Decellularized aortic allografts for aortic valve replacement: same song, second verse?Eur J Cardiothorac Surg. 2024 Jun 3;65(6):ezae197. doi: 10.1093/ejcts/ezae197. Eur J Cardiothorac Surg. 2024. PMID: 38830037 No abstract available.
References
-
- El-Hamamsy I, Laurin C, Williams EE.. The Ross procedure in adolescence and beyond: are there still contraindications? Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2023;26:50–5. - PubMed
-
- Etnel JRG, Grashuis P, Huygens SA, Pekbay B, Papageorgiou G, Helbing WA. et al. The Ross procedure: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes 2018;11:e004748. - PubMed
-
- El-Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L. et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet 2010;376:524–31. - PubMed
-
- Beckmann A, Meyer R, Lewandowski J, Markewitz A, Blaßfeld D, Böning A.. German Heart Surgery Report 2021: the annual updated registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2022;70:362–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

