Lung transcriptomics reveals the underlying mechanism by which aerobic training enhances pulmonary function in chronic obstructive pulmonary disease
- PMID: 38532405
- PMCID: PMC10964526
- DOI: 10.1186/s12890-024-02967-1
Lung transcriptomics reveals the underlying mechanism by which aerobic training enhances pulmonary function in chronic obstructive pulmonary disease
Abstract
Background: Aerobic training is the primary method of rehabilitation for improving respiratory function in patients with chronic obstructive pulmonary disease (COPD) in remission. However, the mechanism underlying this improvement is not yet fully understood. The use of transcriptomics in rehabilitation medicine offers a promising strategy for uncovering the ways in which exercise training improves respiratory dysfunction in COPD patients. In this study, lung tissue was analyzed using transcriptomics to investigate the relationship between exercise and lung changes.
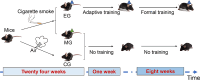
Methods: Mice were exposed to cigarette smoke for 24 weeks, followed by nine weeks of moderate-intensity treadmill exercise, with a control group for comparison. Pulmonary function and structure were assessed at the end of the intervention and RNA sequencing was performed on the lung tissue.
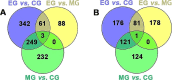
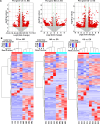
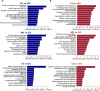
Results: Exercise training was found to improve airway resistance and lung ventilation indices in individuals exposed to cigarette smoke. However, the effect of this treatment on damaged alveoli was weak. The pair-to-pair comparison revealed numerous differentially expressed genes, that were closely linked to inflammation and metabolism.
Conclusions: Further research is necessary to confirm the cause-and-effect relationship between the identified biomarkers and the improvement in pulmonary function, as this was not examined in the present study.
Keywords: Chronic obstructive pulmonary disease; Exercise training; Pulmonary function; Rehabilitation; Transcriptomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Aerobic exercise training engages the canonical wnt pathway to improve pulmonary function and inflammation in COPD.BMC Pulm Med. 2024 May 14;24(1):236. doi: 10.1186/s12890-024-03048-z. BMC Pulm Med. 2024. PMID: 38745304 Free PMC article.
-
Aerobic exercise training does not alter vascular structure and function in chronic obstructive pulmonary disease.Exp Physiol. 2017 Nov 1;102(11):1548-1560. doi: 10.1113/EP086379. Epub 2017 Sep 30. Exp Physiol. 2017. PMID: 28857336 Clinical Trial.
-
Identification of exercise-regulated genes in mice exposed to cigarette smoke.Physiol Rep. 2022 Nov;10(21):e15505. doi: 10.14814/phy2.15505. Physiol Rep. 2022. PMID: 36324300 Free PMC article.
-
Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD012290. doi: 10.1002/14651858.CD012290.pub2. Cochrane Database Syst Rev. 2018. PMID: 30306545 Free PMC article.
-
Rehabilitation effects of land and water-based aerobic exercise on lung function, dyspnea, and exercise capacity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis.Medicine (Baltimore). 2021 Aug 20;100(33):e26976. doi: 10.1097/MD.0000000000026976. Medicine (Baltimore). 2021. PMID: 34414971 Free PMC article.
Cited by
-
An Animal Model of Liuzijue Based on Kinematic Features Exploration: A Pilot Study Conducting in COPD.Immun Inflamm Dis. 2025 Aug;13(8):e70233. doi: 10.1002/iid3.70233. Immun Inflamm Dis. 2025. PMID: 40801240 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

