Association of TRAIL receptor with phosphatase SHP-1 enables repressing T cell receptor signaling and T cell activation through inactivating Lck
- PMID: 38532423
- PMCID: PMC10967194
- DOI: 10.1186/s12929-024-01023-8
Association of TRAIL receptor with phosphatase SHP-1 enables repressing T cell receptor signaling and T cell activation through inactivating Lck
Abstract
Background: T cell receptor (TCR) signaling and T cell activation are tightly regulated by gatekeepers to maintain immune tolerance and avoid autoimmunity. The TRAIL receptor (TRAIL-R) is a TNF-family death receptor that transduces apoptotic signals to induce cell death. Recent studies have indicated that TRAIL-R regulates T cell-mediated immune responses by directly inhibiting T cell activation without inducing apoptosis; however, the distinct signaling pathway that regulates T cell activation remains unclear. In this study, we screened for intracellular TRAIL-R-binding proteins within T cells to explore the novel signaling pathway transduced by TRAIL-R that directly inhibits T cell activation.
Methods: Whole-transcriptome RNA sequencing was used to identify gene expression signatures associated with TRAIL-R signaling during T cell activation. High-throughput screening with mass spectrometry was used to identify the novel TRAIL-R binding proteins within T cells. Co-immunoprecipitation, lipid raft isolation, and confocal microscopic analyses were conducted to verify the association between TRAIL-R and the identified binding proteins within T cells.
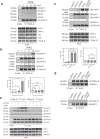
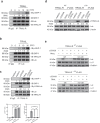
Results: TRAIL engagement downregulated gene signatures in TCR signaling pathways and profoundly suppressed phosphorylation of TCR proximal tyrosine kinases without inducing cell death. The tyrosine phosphatase SHP-1 was identified as the major TRAIL-R binding protein within T cells, using high throughput mass spectrometry-based proteomics analysis. Furthermore, Lck was co-immunoprecipitated with the TRAIL-R/SHP-1 complex in the activated T cells. TRAIL engagement profoundly inhibited phosphorylation of Lck (Y394) and suppressed the recruitment of Lck into lipid rafts in the activated T cells, leading to the interruption of proximal TCR signaling and subsequent T cell activation.
Conclusions: TRAIL-R associates with phosphatase SHP-1 and transduces a unique and distinct immune gatekeeper signal to repress TCR signaling and T cell activation via inactivating Lck. Thus, our results define TRAIL-R as a new class of immune checkpoint receptors for restraining T cell activation, and TRAIL-R/SHP-1 axis can serve as a potential therapeutic target for immune-mediated diseases.
Keywords: Phosphatase; SHP-1; T cell receptor signaling; TRAIL receptor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

