Real-world association between systemic corticosteroid exposure and complications in US patients with severe asthma
- PMID: 38532489
- PMCID: PMC10964513
- DOI: 10.1186/s13223-024-00882-y
Real-world association between systemic corticosteroid exposure and complications in US patients with severe asthma
Abstract
Background: Systemic corticosteroid (SCS) use remains widespread among patients with severe asthma, despite associated complications.
Objective: Evaluate the association between cumulative SCS exposure and SCS-related complications in severe asthma.
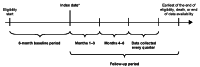
Methods: This retrospective, longitudinal study used claims data from the Optum Clinformatics Data Mart database (GSK ID: 214469). Eligible patients (≥ 12 years old) had an asthma diagnosis and were divided into two cohorts: SCS use and non/burst-SCS use. Patients in the SCS use cohort had a claim for a daily prednisone-equivalent dose ≥ 5 mg SCS following ≥ 6 months of continuous SCS use; those in the non/burst-SCS cohort had no evidence of continuous SCS use and had a non-SCS controller/rescue medication initiation claim. For each cohort, the date of the qualifying claim was the index date. SCS users were further stratified by SCS use during each quarter of follow-up: low (≤ 6 mg/day), medium (> 6-12 mg/day), high (> 12 mg/day), and continuous high (≥ 20 mg/day for 90 days). SCS-related complications were evaluated in the quarter following SCS exposure. The adjusted odds ratios (OR) of experiencing SCS-related complications during follow-up in each of the SCS use groups versus the non/burst SCS cohort were calculated using generalized estimating equations models.
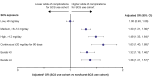
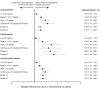
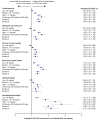
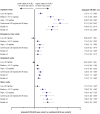
Results: SCS and non/burst-SCS use cohorts included 7473 and 89,281 patients (mean follow-up: 24.6 and 24.2 months), respectively. Compared with the non/burst-SCS use cohort, medium, high, and continuous high SCS use was associated with greater odds of any SCS-related complication (adjusted OR [95% confidence interval]: 1.30 [1.21, 1.39], 1.49 [1.35, 1.64] and 1.63 [1.40, 1.89], respectively) including increased acute gastrointestinal, cardiovascular, and immune system-related complications, and chronic cardiovascular, metabolic/endocrine, central nervous system, bone-/muscle-related, ophthalmologic, and hematologic/oncologic complications. Low-dose SCS use was also associated with significantly increased odds of acute gastrointestinal and immune system-related complications, and chronic bone-/muscle-related and hematologic/oncologic complications versus the non/burst-SCS use cohort.
Conclusion: SCS use, even at low doses, is associated with increased risk of SCS-related complications among patients with severe asthma.
Keywords: Asthma; Cardiovascular; Central nervous system; Endocrine; Gastrointestinal; Metabolic; Ophthalmologic; Systemic corticosteroid; Systemic corticosteroid-related complication.
© 2024. The Author(s).
Conflict of interest statement
TBCa has received consulting and speaking fees from GSK independent of this activity. TCo and AD are GSK employees and hold GSK shares. GG, FL, SDM, JB, and MSD are employees of Analysis Group, which received funding from GSK to complete this study.
Figures
Similar articles
-
Adverse consequences of systemic corticosteroids use among a broad population of US adults with asthma: a real-world analysis.J Med Econ. 2025 Dec;28(1):413-424. doi: 10.1080/13696998.2025.2477877. Epub 2025 Mar 13. J Med Econ. 2025. PMID: 40062655
-
Acute and chronic systemic corticosteroid-related complications in patients with severe asthma.J Allergy Clin Immunol. 2015 Dec;136(6):1488-1495. doi: 10.1016/j.jaci.2015.07.046. Epub 2015 Sep 26. J Allergy Clin Immunol. 2015. PMID: 26414880
-
Burden of illness and costs associated with eosinophilic granulomatosis with polyangiitis: evidence from a managed care database in the United States.J Manag Care Spec Pharm. 2021 Sep;27(9):1249-1259. doi: 10.18553/jmcp.2021.21002. Epub 2021 Jun 24. J Manag Care Spec Pharm. 2021. PMID: 34165321 Free PMC article.
-
Systemic corticosteroids in asthma: A call to action from World Allergy Organization and Respiratory Effectiveness Group.World Allergy Organ J. 2022 Dec 10;15(12):100726. doi: 10.1016/j.waojou.2022.100726. eCollection 2022 Dec. World Allergy Organ J. 2022. PMID: 36582404 Free PMC article. Review.
-
The use of systemic corticosteroids in asthma management in Latin American countries.World Allergy Organ J. 2023 Apr 3;16(4):100760. doi: 10.1016/j.waojou.2023.100760. eCollection 2023 Apr. World Allergy Organ J. 2023. PMID: 37179538 Free PMC article. Review.
References
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention., 2023. Available from: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-2.... [Last accessed August, 2023].
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention., 2006. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2006-GINA.pdf [Last accessed December, 2022].
Grants and funding
LinkOut - more resources
Full Text Sources

