Role of aquaporin-4 polarization in extracellular solute clearance
- PMID: 38532513
- PMCID: PMC10964559
- DOI: 10.1186/s12987-024-00527-7
Role of aquaporin-4 polarization in extracellular solute clearance
Abstract
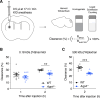
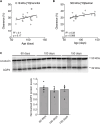
Waste from the brain has been shown to be cleared via the perivascular spaces through the so-called glymphatic system. According to this model the cerebrospinal fluid (CSF) enters the brain in perivascular spaces of arteries, crosses the astrocyte endfoot layer, flows through the parenchyma collecting waste that is subsequently drained along veins. Glymphatic clearance is dependent on astrocytic aquaporin-4 (AQP4) water channels that are highly enriched in the endfeet. Even though the polarized expression of AQP4 in endfeet is thought to be of crucial importance for glymphatic CSF influx, its role in extracellular solute clearance has only been evaluated using non-quantitative fluorescence measurements. Here we have quantitatively evaluated clearance of intrastriatally infused small and large radioactively labeled solutes in mice lacking AQP4 (Aqp4-/-) or lacking the endfoot pool of AQP4 (Snta1-/-). We confirm that Aqp4-/- mice show reduced clearance of both small and large extracellular solutes. Moreover, we find that the Snta1-/- mice have reduced clearance only for the 500 kDa [3H]dextran, but not 0.18 kDa [3H]mannitol suggesting that polarization of AQP4 to the endfeet is primarily important for clearance of large, but not small molecules. Lastly, we observed that clearance of 500 kDa [3H]dextran increased with age in adult mice. Based on our quantitative measurements, we confirm that presence of AQP4 is important for clearance of extracellular solutes, while the perivascular AQP4 localization seems to have a greater impact on clearance of large versus small molecules.
Solute clearance is reduced in mice lacking AQP4 Polarization of AQP4 to the endfeet may have a greater impact on clearance of large versus small molecules Clearance of large but not small solutes is correlated with age within adult age.
Keywords: AQP4; Astrocyte; Dystrophin; Glia; Glymphatic; Syntrophin; Waste clearance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

