Antiretroviral drugs from multiple classes induce loss of excitatory synapses between hippocampal neurons in culture
- PMID: 38533258
- PMCID: PMC10963620
- DOI: 10.3389/fphar.2024.1369757
Antiretroviral drugs from multiple classes induce loss of excitatory synapses between hippocampal neurons in culture
Abstract
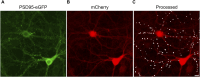
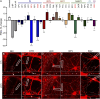
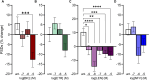
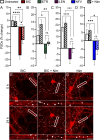
Introduction: Antiretroviral (ARV) drugs have improved prognoses for people living with HIV. However, HIV-associated neurocognitive disorders (HAND) persist despite undetectable viral loads. Some ARVs have been linked to neuropsychiatric effects that may contribute to HAND. Synapse loss correlates with cognitive decline in HAND and synaptic deficits may contribute to the neuropsychiatric effects of ARV drugs. Methods: Using an automated high content assay, rat hippocampal neurons in culture expressing PSD95-eGFP to label glutamatergic synapses and mCherry to fill neuronal structures were imaged before and after treatment with 25 clinically used ARVs. Results and Discussion: At a concentration of 10 μM the protease inhibitors nelfinavir and saquinavir, the non-nucleoside reverse transcriptase inhibitors etravirine and the 8-OH metabolite of efavirenz, the integrase inhibitor bictegravir, and the capsid inhibitor lenacapavir produced synaptic toxicity. Only lenacapavir produced synapse loss at the nanomolar concentrations estimated free in the plasma, although all 4 ARV drugs induced synapse loss at Cmax. Evaluation of combination therapies did not reveal synergistic synaptic toxicity. Synapse loss developed fully by 24 h and persisted for at least 3 days. Bictegravir-induced synapse loss required activation of voltage-gated Ca2+ channels and bictegravir, etravirine, and lenacapavir produced synapse loss by an excitotoxic mechanism. These results indicate that select ARV drugs might contribute to neuropsychiatric effects in combination with drugs that bind serum proteins or in disease states in which synaptic function is altered. The high content imaging assay used here provides an efficient means to evaluate new drugs and drug combinations for potential CNS toxicity.
Keywords: HIV; HIV associated neurocognitive disorder; antiretroviral (ARV); high content imaging (HCI); human immunodeficiency virus; synapse loss.
Copyright © 2024 McMullan, Gansemer and Thayer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

