Biological characteristics of the bacteriophage LDT325 and its potential application against the plant pathogen Pseudomonas syringae
- PMID: 38533332
- PMCID: PMC10964948
- DOI: 10.3389/fmicb.2024.1370332
Biological characteristics of the bacteriophage LDT325 and its potential application against the plant pathogen Pseudomonas syringae
Abstract
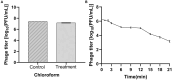
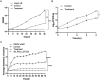
Bud blight disease caused by Pseudomonas syringae is a major bacterial disease of tea plants in China. Concerns regarding the emergence of bacterial resistance to conventional copper controls have indicated the need to devise new methods of disease biocontrol. Phage-based biocontrol may be a sustainable approach to combat bacterial pathogens. In this study, a P. syringae phage was isolated from soil samples. Based on morphological characteristics, bacteriophage vB_PsS_LDT325 belongs to the Siphoviridae family; it has an icosahedral head with a diameter of 53 ± 1 nm and nonretractable tails measuring 110 ± 1 nm. The latent period and burst size of the phage were 10 min and 17 plaque-forming units (PFU)/cell, respectively. Furthermore, an analysis of the biological traits showed that the optimal multiplicity of infection (MOI) of the phage was 0.01. When the temperature exceeded 60°C, the phage titer began to decrease. The phage exhibited tolerance to a wide range of pH (3-11) and maintained relatively stable pH tolerance. It showed a high tolerance to chloroform, but was sensitive to ultraviolet (UV) light. The effects of phage LDT325 in treating P. syringae infections in vivo were evaluated using a tea plant. Plants were inoculated with 2 × 107 colony-forming units (CFU)/mL P. syringae using the needle-prick method and air-dried. Subsequently, plants were inoculated with 2 × 107 PFU/mL LDT325 phage. Compared with control plants, the bacterial count was reduced by 1 log10/0.5 g after 4 days in potted tea plants inoculated with the phage. These results underscore the phage as a potential antibacterial agent for controlling P. syringae.
Keywords: Pseudomonas syringae; bacterial disease; bacteriophage; biocontrol; tea bud blight.
Copyright © 2024 Liu, Wang, Huang, Zhang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Bacteriophage LDT325 enhances Pseudomonas syringae tolerance by improving antioxidant defense in tea plant [Camellia sinensis (L.) O. Kuntze].Front Microbiol. 2025 Jan 7;15:1525040. doi: 10.3389/fmicb.2024.1525040. eCollection 2024. Front Microbiol. 2025. PMID: 39850129 Free PMC article.
-
Efficiency of Phage φ6 for Biocontrol of Pseudomonas syringae pv. syringae: An in Vitro Preliminary Study.Microorganisms. 2019 Aug 23;7(9):286. doi: 10.3390/microorganisms7090286. Microorganisms. 2019. PMID: 31450735 Free PMC article.
-
[Biological characteristics and genomic information of a bacteriophage against pan-drug resistant Klebsiella pneumoniae in a burn patient and its effects on bacterial biofilm].Zhonghua Shao Shang Za Zhi. 2020 Jan 20;36(1):14-23. doi: 10.3760/cma.j.issn.1009-2587.2020.01.004. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32023713 Chinese.
-
Biological and Molecular Characterization of the Lytic Bacteriophage SoKa against Pseudomonas syringae pv. syringae, Causal Agent of Citrus Blast and Black Pit in Tunisia.Viruses. 2022 Sep 2;14(9):1949. doi: 10.3390/v14091949. Viruses. 2022. PMID: 36146756 Free PMC article.
-
Evaluation of several strategies for controlling canker plant disease caused by Pseudomonas syringae.Mol Biol Res Commun. 2025;14(1):1-14. doi: 10.22099/mbrc.2024.51122.2034. Mol Biol Res Commun. 2025. PMID: 39744517 Free PMC article. Review.
Cited by
-
Efficacy of phage vB_Ps_ZCPS13 in controlling Pan-drug-resistant Pseudomonas aeruginosa from urinary tract infections (UTIs) and eradicating biofilms from urinary catheters.Virol J. 2025 Jul 12;22(1):236. doi: 10.1186/s12985-025-02848-x. Virol J. 2025. PMID: 40652263 Free PMC article.
-
Bridging the gap: Phage manufacturing processes from laboratory to agri-food industry.Virus Res. 2025 Mar;353:199537. doi: 10.1016/j.virusres.2025.199537. Epub 2025 Jan 31. Virus Res. 2025. PMID: 39880310 Free PMC article. Review.
References
-
- Balestra G. M., Mazzaglia A., Quattrucci A., Renzi M., Rossetti A. (2009). Current status of bacterial canker spread on kiwifruit in Italy. Aust Plant Dis Notes 4, 34–36. doi: 10.1071/DN09014 - DOI
-
- Bartoli C., Lamichhane J. R., Berge O., Guilbaud C., Varvaro L., Balestra G. M., et al. . (2015). A framework to gauge the epidemic potential of plant pathogens in environmental reservoirs: the example of kiwifruit canker. Mol. Plant Pathol. 16, 137–149. doi: 10.1111/mpp.12167, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

