Diving into the zebrafish brain: exploring neuroscience frontiers with genetic tools, imaging techniques, and behavioral insights
- PMID: 38533456
- PMCID: PMC10963419
- DOI: 10.3389/fnmol.2024.1358844
Diving into the zebrafish brain: exploring neuroscience frontiers with genetic tools, imaging techniques, and behavioral insights
Abstract
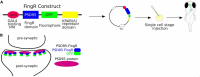
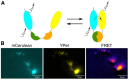
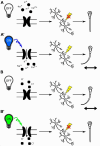
The zebrafish (Danio rerio) is increasingly used in neuroscience research. Zebrafish are relatively easy to maintain, and their high fecundity makes them suitable for high-throughput experiments. Their small, transparent embryos and larvae allow for easy microscopic imaging of the developing brain. Zebrafish also share a high degree of genetic similarity with humans, and are amenable to genetic manipulation techniques, such as gene knockdown, knockout, or knock-in, which allows researchers to study the role of specific genes relevant to human brain development, function, and disease. Zebrafish can also serve as a model for behavioral studies, including locomotion, learning, and social interactions. In this review, we present state-of-the-art methods to study the brain function in zebrafish, including genetic tools for labeling single neurons and neuronal circuits, live imaging of neural activity, synaptic dynamics and protein interactions in the zebrafish brain, optogenetic manipulation, and the use of virtual reality technology for behavioral testing. We highlight the potential of zebrafish for neuroscience research, especially regarding brain development, neuronal circuits, and genetic-based disorders and discuss its certain limitations as a model.
Keywords: behavioral studies; brain development; brain imaging; genetic tools; modern methods for neuroscience; optogenetics; virtual reality; zebrafish.
Copyright © 2024 Doszyn, Dulski and Zmorzynska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

