Cellular interactions in tumor microenvironment during breast cancer progression: new frontiers and implications for novel therapeutics
- PMID: 38533507
- PMCID: PMC10963559
- DOI: 10.3389/fimmu.2024.1302587
Cellular interactions in tumor microenvironment during breast cancer progression: new frontiers and implications for novel therapeutics
Abstract
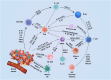
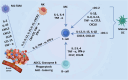
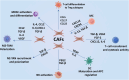
The breast cancer tumor microenvironment (TME) is dynamic, with various immune and non-immune cells interacting to regulate tumor progression and anti-tumor immunity. It is now evident that the cells within the TME significantly contribute to breast cancer progression and resistance to various conventional and newly developed anti-tumor therapies. Both immune and non-immune cells in the TME play critical roles in tumor onset, uncontrolled proliferation, metastasis, immune evasion, and resistance to anti-tumor therapies. Consequently, molecular and cellular components of breast TME have emerged as promising therapeutic targets for developing novel treatments. The breast TME primarily comprises cancer cells, stromal cells, vasculature, and infiltrating immune cells. Currently, numerous clinical trials targeting specific TME components of breast cancer are underway. However, the complexity of the TME and its impact on the evasion of anti-tumor immunity necessitate further research to develop novel and improved breast cancer therapies. The multifaceted nature of breast TME cells arises from their phenotypic and functional plasticity, which endows them with both pro and anti-tumor roles during tumor progression. In this review, we discuss current understanding and recent advances in the pro and anti-tumoral functions of TME cells and their implications for developing safe and effective therapies to control breast cancer progress.
Keywords: breast cancer; immune cells; metastasis; stroma; tumor microenvironment.
Copyright © 2024 Akinsipe, Mohamedelhassan, Akinpelu, Pondugula, Mistriotis, Avila and Suryawanshi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Tumor microenvironment: recent advances in understanding and its role in modulating cancer therapies.Med Oncol. 2025 Mar 18;42(4):117. doi: 10.1007/s12032-025-02641-4. Med Oncol. 2025. PMID: 40102282 Review.
-
The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: New challenges and therapeutic opportunities.Cell Oncol (Dordr). 2021 Dec;44(6):1209-1229. doi: 10.1007/s13402-021-00634-9. Epub 2021 Sep 16. Cell Oncol (Dordr). 2021. PMID: 34528143 Review.
-
Disruption of Cell-Cell Communication in Anaplastic Thyroid Cancer as an Immunotherapeutic Opportunity.Adv Exp Med Biol. 2021;1350:33-66. doi: 10.1007/978-3-030-83282-7_2. Adv Exp Med Biol. 2021. PMID: 34888843
-
Taking a Full Snapshot of Cancer Biology: Deciphering the Tumor Microenvironment for Effective Cancer Therapy in the Oncology Clinic.OMICS. 2020 Apr;24(4):175-179. doi: 10.1089/omi.2020.0019. Epub 2020 Mar 13. OMICS. 2020. PMID: 32176591 Review.
-
Tumor microenvironment signaling and therapeutics in cancer progression.Cancer Commun (Lond). 2023 May;43(5):525-561. doi: 10.1002/cac2.12416. Epub 2023 Apr 2. Cancer Commun (Lond). 2023. PMID: 37005490 Free PMC article. Review.
Cited by
-
Contemporary Approaches to Immunotherapy of Solid Tumors.Cancers (Basel). 2024 Jun 19;16(12):2270. doi: 10.3390/cancers16122270. Cancers (Basel). 2024. PMID: 38927974 Free PMC article. Review.
-
The balance between N1 and N2 neutrophils implications for breast cancer immunotherapy: a narrative review.Ann Med Surg (Lond). 2025 May 12;87(6):3682-3690. doi: 10.1097/MS9.0000000000003361. eCollection 2025 Jun. Ann Med Surg (Lond). 2025. PMID: 40486616 Free PMC article. Review.
-
Multimodal Phasor Approach to Study Breast Cancer Cell Invasion in a 3D Spheroid Model.Chem Biomed Imaging. 2025 May 15;3(7):433-442. doi: 10.1021/cbmi.5c00021. eCollection 2025 Jul 28. Chem Biomed Imaging. 2025. PMID: 40747157 Free PMC article.
-
The Tumour Microenvironment and Epigenetic Regulation in BRCA1 Pathogenic Variant-Associated Breast Cancers.Cancers (Basel). 2024 Nov 21;16(23):3910. doi: 10.3390/cancers16233910. Cancers (Basel). 2024. PMID: 39682099 Free PMC article. Review.
-
Neutrophils in Ocular Diseases.Int J Mol Sci. 2024 Jul 15;25(14):7736. doi: 10.3390/ijms25147736. Int J Mol Sci. 2024. PMID: 39062975 Free PMC article. Review.
References
-
- Organization WH. Cancer IAfRo. Organization WH . Global cancer observatory. (2020).
-
- Mohammed EA, Solyman MTM, Omar NN, Hasan NMA. Imaging features of breast cancer molecular subtypes: An Updated Review of the Literature. SVU-International J Med Sci. (2022) 5:92–103. doi: 10.21608/svuijm.2021.104214.1238 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

