Role of CD38 in anti-tumor immunity of small cell lung cancer
- PMID: 38533509
- PMCID: PMC10963403
- DOI: 10.3389/fimmu.2024.1348982
Role of CD38 in anti-tumor immunity of small cell lung cancer
Abstract
Introduction: Immune checkpoint blockade (ICB) with or without chemotherapy has a very modest benefit in patients with small cell lung cancer (SCLC). SCLC tumors are characterized by high tumor mutation burden (TMB) and low PD-L1 expression. Therefore, TMB and PD-L1 do not serve as biomarkers of ICB response in SCLC. CD38, a transmembrane glycoprotein, mediates immunosuppression in non-small cell lung cancer (NSCLC). In this brief report, we highlight the potential role of CD38 as a probable biomarker of immunotherapy response in SCLC.
Methods: We evaluated the role of CD38 as a determinant of tumor immune microenvironment in SCLC with bulk and single-cell transcriptomic analyses and protein assessments of clinical samples and preclinical models, including CD38 in vivo blockade.
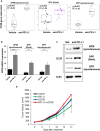
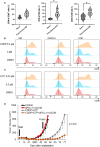
Results: In SCLC clinical samples, CD38 levels were significantly correlated with the gene expression of the immunosuppressive markers FOXP3, PD-1 and CTLA-4. CD38 expression was significantly enhanced after chemotherapy and ICB treatment in SCLC preclinical models and clinical samples. A combination of cisplatin/etoposide, ICB, and CD38 blockade delayed tumor growth compared to cisplatin/etoposide.
Conclusion: Our study provides a preliminary but important direction toward exploring CD38 as a potential biomarker of ICB response and CD38 blockade as a combination strategy for chemo-immunotherapy in SCLC.
Keywords: CD38; immune checkpoint blockade; lung cancer; resistance; small cell lung cancer.
Copyright © 2024 Taniguchi, Chavan, Chow, Chan, Mukae, Rudin and Sen.
Conflict of interest statement
TS has grant funding from Jazz Pharmaceuticals. CR has consulted regarding oncology drug development with AbbVie, Amgen, Astra Zeneca, D2G, Daiichi Sankyo, Epizyme, Genentech/Roche, Ipsen, Jazz, Kowa, Lilly, Merck, and Syros. He serves on the scientific advisory boards of Auron, Bridge Medicines, DISCO, Earli, and Harpoon Therapeutics. JC has equity in Mirati Therapeutics and the provision of services from Sonata Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. . CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. (2018) 8 (9):1156–75. doi: 10.1158/2159-8290.CD-17-1033 - DOI - PMC - PubMed
-
- Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. . Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17 (7):883–95. doi: 10.1016/S1470-2045(16)30098-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

