Activin E is a transforming growth factor β ligand that signals specifically through activin receptor-like kinase 7
- PMID: 38533769
- PMCID: PMC11088876
- DOI: 10.1042/BCJ20230404
Activin E is a transforming growth factor β ligand that signals specifically through activin receptor-like kinase 7
Abstract
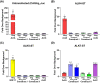
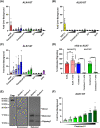
Activins are one of the three distinct subclasses within the greater Transforming growth factor β (TGFβ) superfamily. First discovered for their critical roles in reproductive biology, activins have since been shown to alter cellular differentiation and proliferation. At present, members of the activin subclass include activin A (ActA), ActB, ActC, ActE, and the more distant members myostatin and GDF11. While the biological roles and signaling mechanisms of most activins class members have been well-studied, the signaling potential of ActE has remained largely unknown. Here, we characterized the signaling capacity of homodimeric ActE. Molecular modeling of the ligand:receptor complexes showed that ActC and ActE shared high similarity in both the type I and type II receptor binding epitopes. ActE signaled specifically through ALK7, utilized the canonical activin type II receptors, ActRIIA and ActRIIB, and was resistant to the extracellular antagonists follistatin and WFIKKN. In mature murine adipocytes, ActE invoked a SMAD2/3 response via ALK7, like ActC. Collectively, our results establish ActE as a specific signaling ligand which activates the type I receptor, ALK7.
Keywords: adipocytes; molecular mechanisms; receptor tyrosine kinases; transforming growth factors.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Update of
-
Activin E is a TGFβ ligand that signals specifically through activin receptor-like kinase 7.bioRxiv [Preprint]. 2023 Sep 25:2023.09.25.559288. doi: 10.1101/2023.09.25.559288. bioRxiv. 2023. Update in: Biochem J. 2024 Apr 10;481(7):547-564. doi: 10.1042/BCJ20230404. PMID: 37808681 Free PMC article. Updated. Preprint.
References
-
- Walton, K.L., Makanji, Y., Wilce, M.C., Chan, K.L., Robertson, D.M. and Harrison, C.A. (2009) A common biosynthetic pathway governs the dimerization and secretion of inhibin and related transforming growth factor beta (TGFbeta) ligands. J. Biol. Chem. 284, 9311–9320 10.1074/jbcM808763200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

