The Effect of an Electronic Medical Record-Based Clinical Decision Support System on Adherence to Clinical Protocols in Inflammatory Bowel Disease Care: Interrupted Time Series Study
- PMID: 38533825
- PMCID: PMC11004614
- DOI: 10.2196/55314
The Effect of an Electronic Medical Record-Based Clinical Decision Support System on Adherence to Clinical Protocols in Inflammatory Bowel Disease Care: Interrupted Time Series Study
Abstract
Background: Clinical decision support systems (CDSSs) embedded in electronic medical records (EMRs), also called electronic health records, have the potential to improve the adoption of clinical guidelines. The University of Alberta Inflammatory Bowel Disease (IBD) Group developed a CDSS for patients with IBD who might be experiencing disease flare and deployed it within a clinical information system in 2 continuous time periods.
Objective: This study aims to evaluate the impact of the IBD CDSS on the adherence of health care providers (ie, physicians and nurses) to institutionally agreed clinical management protocols.
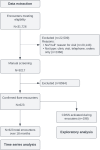
Methods: A 2-period interrupted time series (ITS) design, comparing adherence to a clinical flare management protocol during outpatient visits before and after the CDSS implementation, was used. Each interruption was initiated with user training and a memo with instructions for use. A group of 7 physicians, 1 nurse practitioner, and 4 nurses were invited to use the CDSS. In total, 31,726 flare encounters were extracted from the clinical information system database, and 9217 of them were manually screened for inclusion. Each data point in the ITS analysis corresponded to 1 month of individual patient encounters, with a total of 18 months of data (9 before and 9 after interruption) for each period. The study was designed in accordance with the Statement on Reporting of Evaluation Studies in Health Informatics (STARE-HI) guidelines for health informatics evaluations.
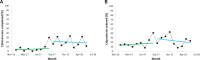
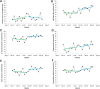
Results: Following manual screening, 623 flare encounters were confirmed and designated for ITS analysis. The CDSS was activated in 198 of 623 encounters, most commonly in cases where the primary visit reason was a suspected IBD flare. In Implementation Period 1, before-and-after analysis demonstrates an increase in documentation of clinical scores from 3.5% to 24.1% (P<.001), with a statistically significant level change in ITS analysis (P=.03). In Implementation Period 2, the before-and-after analysis showed further increases in the ordering of acute disease flare lab tests (47.6% to 65.8%; P<.001), including the biomarker fecal calprotectin (27.9% to 37.3%; P=.03) and stool culture testing (54.6% to 66.9%; P=.005); the latter is a test used to distinguish a flare from an infectious disease. There were no significant slope or level changes in ITS analyses in Implementation Period 2. The overall provider adoption rate was moderate at approximately 25%, with greater adoption by nurse providers (used in 30.5% of flare encounters) compared to physicians (used in 6.7% of flare encounters).
Conclusions: This is one of the first studies to investigate the implementation of a CDSS for IBD, designed with a leading EMR software (Epic Systems), providing initial evidence of an improvement over routine care. Several areas for future research were identified, notably the effect of CDSSs on outcomes and how to design a CDSS with greater utility for physicians. CDSSs for IBD should also be evaluated on a larger scale; this can be facilitated by regional and national centralized EMR systems.
Keywords: CDSS; EHR; EHRs; EMR; EMRs; IBD; adherence; bowel; chart; clinical; clinical practice guidelines; decision support; decision support system; electronic chart; electronic health records; electronic medical chart; electronic medical records; flare; flares; gastroenterology; gastrointestinal; health record; implementation; implementation science; inflammatory bowel disease; internal medicine; interrupted time series analysis; medical record; nurse; standardized care; steroid; steroids; time series.
© Reed Taylor Sutton, Kaitlyn Delaney Chappell, David Pincock, Daniel Sadowski, Daniel C Baumgart, Karen Ivy Kroeker. Originally published in JMIR Medical Informatics (https://medinform.jmir.org).
Conflict of interest statement
Figures


Similar articles
-
Benefits of Clinical Decision Support Systems for the Management of Noncommunicable Chronic Diseases: Targeted Literature Review.Interact J Med Res. 2024 Nov 27;13:e58036. doi: 10.2196/58036. Interact J Med Res. 2024. PMID: 39602213 Free PMC article. Review.
-
Human-Centered Design of a Clinical Decision Support for Anemia Screening in Children with Inflammatory Bowel Disease.Appl Clin Inform. 2023 Mar;14(2):345-353. doi: 10.1055/a-2040-0578. Epub 2023 Feb 21. Appl Clin Inform. 2023. PMID: 36809791 Free PMC article.
-
Availability and usage of clinical decision support systems (CDSSs) in office-based primary care settings in the USA.BMJ Health Care Inform. 2019 Dec;26(1):e100015. doi: 10.1136/bmjhci-2019-100015. BMJ Health Care Inform. 2019. PMID: 31818828 Free PMC article.
-
Decision support systems in clinical practice: The case of venous thromboembolism prevention.Int J Risk Saf Med. 2015;27 Suppl 1:S104-5. doi: 10.3233/JRS-150709. Int J Risk Saf Med. 2015. PMID: 26639683
-
Using Computer Alert Systems in the Emergency Room to Screen for Child Abuse [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. PMID: 38598650 Free Books & Documents. Review.
Cited by
-
A framework for clinical care pathway renewal: an example from inflammatory bowel disease.BMC Med Res Methodol. 2025 Jul 9;25(1):173. doi: 10.1186/s12874-025-02616-z. BMC Med Res Methodol. 2025. PMID: 40634845 Free PMC article.
-
Germany and Europe lead digital innovation and AI with collaborative health data use at continental level.NPJ Digit Med. 2025 Apr 20;8(1):215. doi: 10.1038/s41746-025-01631-0. NPJ Digit Med. 2025. PMID: 40254642 Free PMC article.
-
Beirut Port Blast: Use of Electronic Health Record System During a Mass Casualty Event.West J Emerg Med. 2025 Jan;26(1):20-29. doi: 10.5811/westjem.20942. West J Emerg Med. 2025. PMID: 39918138 Free PMC article.
References
-
- Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000;(1):65–70. Medline. - PubMed
-
- Shortliffe T. Medical thinking: what should we do?. Conference on Medical Thinking; Jun 23, 2006; London, UK. [05-03-2024]. Presented at. URL. Accessed.
LinkOut - more resources
Full Text Sources
Miscellaneous

