Targeting intracellular nontuberculous mycobacteria and M. tuberculosis with a bactericidal enzymatic cocktail
- PMID: 38534149
- PMCID: PMC11064574
- DOI: 10.1128/spectrum.03534-23
Targeting intracellular nontuberculous mycobacteria and M. tuberculosis with a bactericidal enzymatic cocktail
Abstract
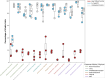
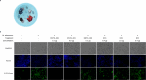
To address intracellular mycobacterial infections, we developed a cocktail of four enzymes that catalytically attack three layers of the mycobacterial envelope. This cocktail is delivered to macrophages, through a targeted liposome presented here as ENTX_001. Endolytix Cocktail 1 (EC1) leverages mycobacteriophage lysin enzymes LysA and LysB, while also including α-amylase and isoamylase for degradation of the mycobacterial envelope from outside of the cell. The LysA family of proteins from mycobacteriophages has been shown to cleave the peptidoglycan layer, whereas LysB is an esterase that hydrolyzes the linkage between arabinogalactan and mycolic acids of the mycomembrane. The challenge of gaining access to the substrates of LysA and LysB provided exogenously was addressed by adding amylase enzymes that degrade the extracellular capsule shown to be present in Mycobacterium tuberculosis. This enzybiotic approach avoids antimicrobial resistance, specific receptor-mediated binding, and intracellular DNA surveillance pathways that limit many bacteriophage applications. We show this cocktail of enzymes is bactericidal in vitro against both rapid- and slow-growing nontuberculous mycobacteria (NTM) as well as M. tuberculosis strains. The EC1 cocktail shows superior killing activity when compared to previously characterized LysB alone. EC1 is also powerfully synergistic with standard-of-care antibiotics. In addition to in vitro killing of NTM, ENTX_001 demonstrates the rescue of infected macrophages from necrotic death by Mycobacteroides abscessus and Mycobacterium avium. Here, we demonstrate shredding of mycobacterial cells by EC1 into cellular debris as a mechanism of bactericide.IMPORTANCEThe world needs entirely new forms of antibiotics as resistance to chemical antibiotics is a critical problem facing society. We addressed this need by developing a targeted enzyme therapy for a broad range of species and strains within mycobacteria and highly related genera including nontuberculous mycobacteria such as Mycobacteroides abscessus, Mycobacterium avium, Mycobacterium intracellulare, as well as Mycobacterium tuberculosis. One advantage of this approach is the ability to drive our lytic enzymes through encapsulation into macrophage-targeted liposomes resulting in attack of mycobacteria in the cells that harbor them where they hide from the adaptive immune system and grow. Furthermore, this approach shreds mycobacteria independent of cell physiology as the drug targets the mycobacterial envelope while sidestepping the host range limitations observed with phage therapy and resistance to chemical antibiotics.
Keywords: AMR; LysA; LysB; NTM; arabinogalactan; capsule; enzybiotic; liposome; macrophage; mycobacteriophage; mycomembrane; peptidoglycan; phagolysosome.
Conflict of interest statement
All Endolytix employees may hold stock and/or stock options.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

