Competitive Immunoassay in a Microfluidic Biochip for In-Field Detection of Abscisic Acid in Grapes
- PMID: 38534230
- PMCID: PMC10968099
- DOI: 10.3390/bios14030123
Competitive Immunoassay in a Microfluidic Biochip for In-Field Detection of Abscisic Acid in Grapes
Abstract
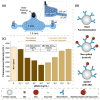
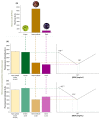
Viticulture and associated products are an important part of the economy in many countries. However, biotic and abiotic stresses impact negatively the production of grapes and wine. Climate change is in many aspects increasing both these stresses. Routine sample retrievals and analysis tend to be time-consuming and require expensive equipment and skilled personnel to operate. These challenges could be overcome through the development of a miniaturized analytic device for early detection of grapevine stresses in the field. Abscisic acid is involved in several plant processes, including the onset of fruit ripening and tolerance mechanisms against drought stress. This hormone can be detected through a competitive immunoassay and is found in plants in concentrations up to 10-1 mg/mL. A microfluidic platform is developed in this work which can detect a minimum of 10-11 mg/mL of abscisic acid in buffer. Grape samples were tested using the microfluidic system alongside benchmark techniques such as high-performance liquid chromatography. The microfluidic system could detect the increase to 10-5 mg/mL of abscisic acid present in real berry samples at the veraison stage of ripening.
Keywords: abscisic acid; agriculture; biosensing; competitive immunoassay; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Causas Das Alterações Climáticas. [(accessed on 9 November 2023)]. Available online: https://climate.ec.europa.eu/climate-change/causes-climate-change_pt.
-
- Lo Presti D., Di Tocco J., Massaroni C., Cimini S., De Gara L., Singh S., Raucci A., Manganiello G., Woo S.L., Schena E., et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023;222:115005. doi: 10.1016/j.bios.2022.115005. - DOI - PubMed
-
- OIV 2019 Statistical Report on World Vitiviniculture. [(accessed on 30 July 2023)]. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-wo....
-
- Li J., Li C., Smith S.M. Hormone Metabolism and Signaling in Plants. Acdemic Press; Cambridge, MA, USA: 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

