Plasmonic Fluorescence Sensors in Diagnosis of Infectious Diseases
- PMID: 38534237
- PMCID: PMC10967809
- DOI: 10.3390/bios14030130
Plasmonic Fluorescence Sensors in Diagnosis of Infectious Diseases
Abstract
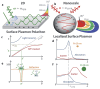
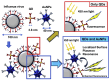
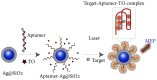
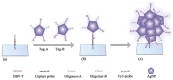
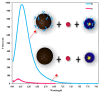
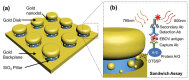
The increasing demand for rapid, cost-effective, and reliable diagnostic tools in personalized and point-of-care medicine is driving scientists to enhance existing technology platforms and develop new methods for detecting and measuring clinically significant biomarkers. Humanity is confronted with growing risks from emerging and recurring infectious diseases, including the influenza virus, dengue virus (DENV), human immunodeficiency virus (HIV), Ebola virus, tuberculosis, cholera, and, most notably, SARS coronavirus-2 (SARS-CoV-2; COVID-19), among others. Timely diagnosis of infections and effective disease control have always been of paramount importance. Plasmonic-based biosensing holds the potential to address the threat posed by infectious diseases by enabling prompt disease monitoring. In recent years, numerous plasmonic platforms have risen to the challenge of offering on-site strategies to complement traditional diagnostic methods like polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays (ELISA). Disease detection can be accomplished through the utilization of diverse plasmonic phenomena, such as propagating surface plasmon resonance (SPR), localized SPR (LSPR), surface-enhanced Raman scattering (SERS), surface-enhanced fluorescence (SEF), surface-enhanced infrared absorption spectroscopy, and plasmonic fluorescence sensors. This review focuses on diagnostic methods employing plasmonic fluorescence sensors, highlighting their pivotal role in swift disease detection with remarkable sensitivity. It underscores the necessity for continued research to expand the scope and capabilities of plasmonic fluorescence sensors in the field of diagnostics.
Keywords: LSPR; SPR; biosensors; fluorescence; infectious disease; plasmonics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
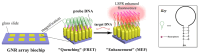






Similar articles
-
Recent Progress in Plasmonic Biosensing Schemes for Virus Detection.Sensors (Basel). 2020 Aug 22;20(17):4745. doi: 10.3390/s20174745. Sensors (Basel). 2020. PMID: 32842601 Free PMC article. Review.
-
Plasmonic-based platforms for diagnosis of infectious diseases at the point-of-care.Biotechnol Adv. 2019 Dec;37(8):107440. doi: 10.1016/j.biotechadv.2019.107440. Epub 2019 Aug 30. Biotechnol Adv. 2019. PMID: 31476421 Review.
-
Plasmonic nanosensors for pharmaceutical and biomedical analysis.J Pharm Biomed Anal. 2023 Nov 30;236:115671. doi: 10.1016/j.jpba.2023.115671. Epub 2023 Aug 22. J Pharm Biomed Anal. 2023. PMID: 37659267 Review.
-
A comprehensive review on plasmonic-based biosensors used in viral diagnostics.Commun Biol. 2021 Jan 15;4(1):70. doi: 10.1038/s42003-020-01615-8. Commun Biol. 2021. PMID: 33452375 Free PMC article. Review.
-
Lateral flow immunoassay based on surface-enhanced Raman scattering using pH-induced phage-templated hierarchical plasmonic assembly for point-of-care diagnosis of infectious disease.Biosens Bioelectron. 2024 Apr 15;250:116061. doi: 10.1016/j.bios.2024.116061. Epub 2024 Jan 23. Biosens Bioelectron. 2024. PMID: 38278123
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

